AF is the most common cardiac arrhythmia of clinical significance with an estimated prevalence of >33 million individuals globally.1 AF can be associated with significant symptoms and impaired quality of life of affected patients while also increasing the risk of stroke, heart failure and death.2 AF frequently co-exists with heart failure (HF). Up to half of patients with HF in the Framingham Heart Study developed AF, while HF occurred in more than one-third of individuals with AF.3 Initial studies of rhythm control versus rate control to treat AF demonstrated equivalent outcomes, but rhythm control was pursued with electrical cardioversion or anti-arrhythmic medications associated with limited efficacy and adverse effects.4,5 Safety concerns have also been raised with anti-arrhythmic drugs with some medications independently associated with higher mortality rates.6,7
Catheter ablation has emerged as an effective treatment strategy in patients with AF and HF with observational studies, randomised controlled trials (RCTs) and meta-analyses all demonstrating clinical improvements compared with rate-control strategies.8–12 However, there remain unanswered questions as to which group of patients with HF might benefit the most from catheter ablation, the optimal ablation strategy in this population and when to pursue ablation. In this state-of-the-art review, we assess the evidence from RCTs published within the last 10 years as well as additional observational and mechanistic studies to clarify what remains unknown and what could be a priority for future investigation.
Current Guidelines for Management
In patients who have co-existing AF and HF, the main aims of treatment are to prevent adverse outcomes, improve symptoms and maintain a good quality of life. The 2016 European Society of Cardiology guidelines on the management of AF state that the ‘indications for catheter ablation in HF patients with reduced ejection fraction (HFrEF) should be carefully balanced and procedures performed in experienced centres.’13 The guidelines also recognise that AF ablation can be more demanding in this patient cohort compared with patients without HF. In patients presenting acutely with AF and HF, the guidelines recommend focusing on normalising fluid balance, aiming for an initial heart rate target of <110 bpm, use of anticoagulation, inhibition of the renin–angiotensin–aldosterone system and early consideration of rhythm control.13
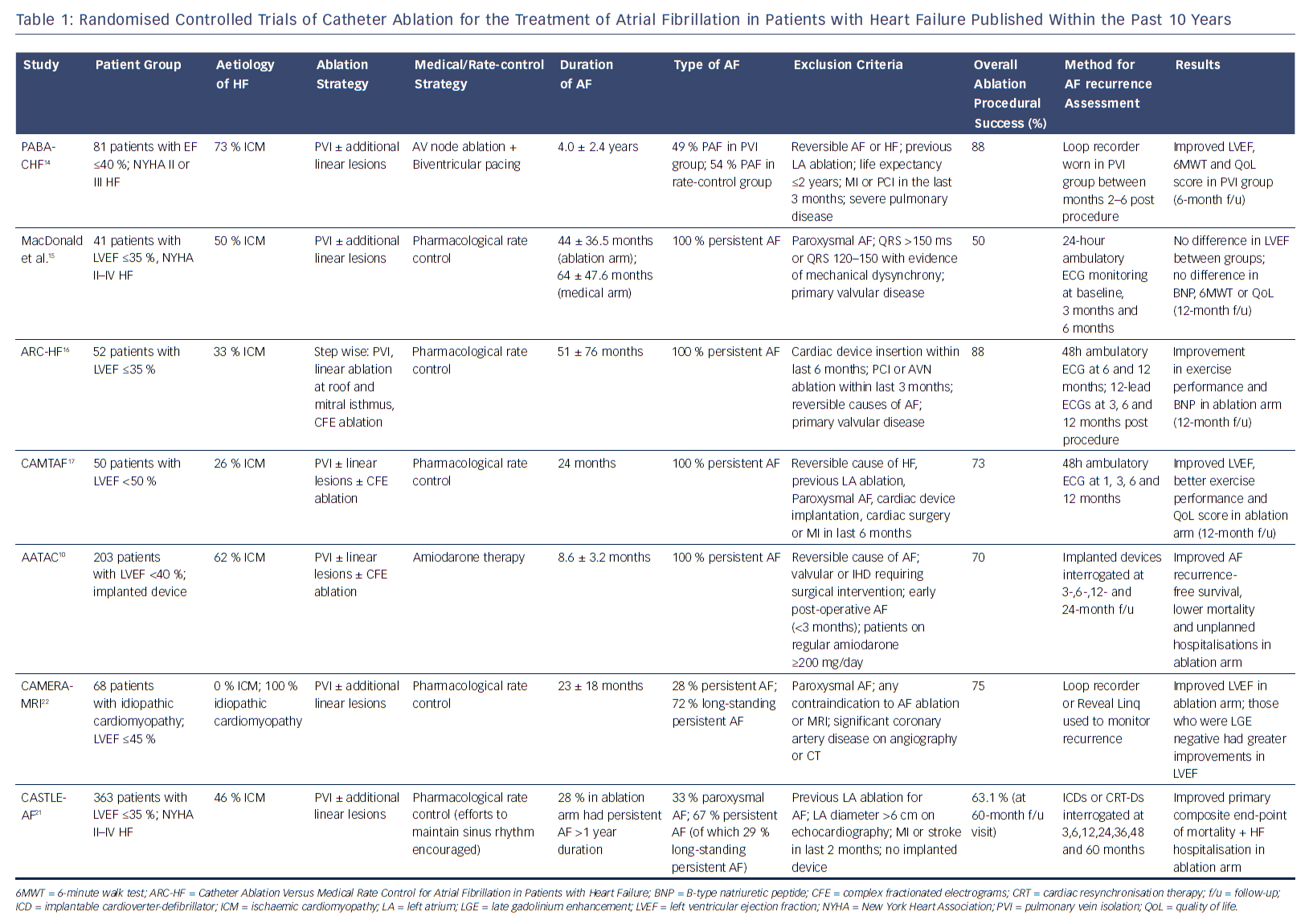
However, there is no clear consensus on which patients with HF should be offered catheter ablation or the optimal ablation strategy in this setting. A growing number of studies have now been published to assess the effectiveness of catheter ablation on improving clinical outcomes in patients with AF and HF (Table 1). These studies have challenged previous treatment paradigms in which rate control was considered equivalent to rhythm control in this patient population.
Clinical Trials of Catheter Ablation in Patients with AF and Heart Failure
Early trials of catheter ablation versus rate control therapy for patients with AF and HF included small numbers of patients and were not adequately powered to assess hard endpoints such as mortality. In the Pulmonary Vein Antrum Isolation versus AV node Ablation with Bi-Ventricular Pacing for Treatment of Atrial Fibrillation in patients with Congestive Heart Failure (PABA-CHF) study, 81 patients with drug-resistant AF and an EF <40 % were randomised to undergo either AF ablation with pulmonary vein isolation (PVI) or AV node ablation with biventricular pacing.14 Additional linear lesions as well as targeting of complex fractionated electrograms (CFE) were allowed according to the preference of the centre and operator. Patients who underwent PVI showed improvements in the composite primary endpoint including EF measurement with echocardiography, 6-minute walk test and a quality of life score compared with AV node ablation with biventricular pacing. The majority of patients in both arms of the study had ischaemic cardiomyopathy (73 % and 68 %, respectively) and a mean duration of AF of 4 years. In the ablation group, 51 % had persistent or long-standing persistent AF where clinical outcomes are known to be inferior to paroxysmal AF. Intriguingly, those patients who underwent AV node ablation and biventricular pacing did not demonstrate more significant improvements in their LVF (EF 28 %), although this group of patients appeared to have narrow QRS intervals at baseline (90 ± 10 ms).14
In a smaller study (41 patients), MacDonald et al. found no difference in EF measured using cardiac MRI in patients undergoing catheter ablation versus medical rate control at 6-month follow-up.15 In this study, only patients with persistent AF were included, while mean LVEF measured at baseline was only 16.1 % in the ablation group and 19.6 % in the medical group, suggesting more advanced disease. Around 90 % of patients were in New York Heart Association (NYHA) class III or above. Furthermore, only 50 % of patients in the ablation group maintained sinus rhythm at the end of the study. Although the study was likely underpowered to detect a difference in the primary endpoint, it does raise the importance of careful consideration of the likelihood of success in specific groups of patients with AF and HF.15 In a subsequent study (ARC-HF), with an improvement in maintenance of sinus rhythm and higher single-procedure success rate, catheter ablation led to an increase in peak oxygen consumption compared with rate control.16 The Catheter Ablation Versus Medical Treatment of Atrial Fibrillation (CAMTAF) trial included a higher proportion of patients with non-ischaemic cardiomyopathy (74 %) compared to previous trials, while 92 % of all patients in the study had long-standing persistent AF.17 In addition, those patients who were thought to be clearly symptomatic from AF were excluded, as the aim of the study was to use ablation to treat HF rather than symptomatic refractory AF. An improvement in LVEF, peak oxygen consumption and quality of life score was seen in the ablation arm versus medical rate control. Interestingly, the duration of continuous AF was significantly lower in the CAMTAF study compared with that in the study by MacDonald et al.13 (24 months versus 53 months).17
In these early RCTs, the duration of follow-up ranged 6–10 months. Meanwhile, the single-procedure success rates of catheter ablation ranged 38–71 %, with overall success rates ranging 50–88 %. Whether catheter ablation maintained sinus rhythm in the long term is unclear based on these early results. The ablation strategy was also heterogeneous with all patients undergoing at least PVI, but a large proportion had additional linear lesions dependant on the operator and centre. A meta-analysis of these trials revealed that an improvement in LVEF was the most consistent benefit in functional outcome with a mean difference of 8.53 % (95 % CI [6.40–10.67]).11 This raises the issue of whether catheter ablation would improve functional outcome in patients with HF and preserved EF (HFpEF) with limited data that suggest that these patients may be older, have more co-morbidities, be more commonly female and may have higher procedural complications.18,19 However, a recent study of 230 patients found no differences in arrhythmia-free recurrence, NYHA functional class and procedural characteristics between HFpEF and HFrEF patients undergoing AF ablation.20 This was a single-centre, retrospective study and further research is needed to assess the role of catheter ablation in HFpEF.
The early RCTs included small numbers of patients and were therefore adequately powered only to assess surrogate end-points such as ejection fraction, exercise capacity and quality of life. More recently a number of highly anticipated trials have now been published assessing hard end-points such as mortality. In the Ablation vs. Amiodarone for Treatment of Atrial Fibrillation in Patients with Congestive Heart Failure and an Implanted ICD/CRTD (AATAC) study, patients with persistent AF, LVEF <40 % and NYHA Class II–III heart failure were randomised to either receive catheter ablation or amiodarone.10 Unlike previous RCTs, the aim in both arms was rhythm control with a primary endpoint of AF recurrence and secondary endpoints including all-cause mortality and unplanned hospitalisation. Interestingly, catheter ablation was superior to amiodarone in achieving freedom from AF recurrence as well reducing all-cause mortality and unplanned hospitalisation rates. During a longer 24-month follow-up period, 70 % of patients in the ablation arm (95 % CI [60–78]) and 34 % in the control arm (95 % CI [25–44]) remained arrhythmia-free. The duration of AF at randomisation was significantly shorter than in previous studies (8.6 months in the ablation arm and 8.4 months in the amiodarone arm), while all patients had an implanted device, which strengthened the quality of the outcome data. A lower all-cause mortality rate (secondary endpoint) was also reported in the catheter ablation arm (8 % versus 18 %; p=0.037).
Although promising, it should be noted that the AATAC study compared ablation with a drug known to have significant toxicities; 10.4 % of treatment failures in the amiodarone group had the drug withdrawn due to adverse effects. The study does, however, raise the importance of early treatment before long-standing persistent AF develops.10
In this context, the Catheter Ablation versus Standard Conventional Therapy in Patients with Left Ventricular Dysfunction and Atrial Fibrillation (CASTLE-AF) trial assessed the impact of ablation on mortality and HF progression rates.21 In this study, 363 patients with symptomatic paroxysmal or persistent AF, NYHA II–IV heart failure, LVEF <35 % and an implanted device were randomised to receive either catheter ablation or medical therapy (with rhythm control encouraged). Over a median follow-up of 37.8 months, the primary composite endpoint of death from any cause or hospitalisation due to HF was significantly less frequent in the ablation group than the medical therapy group (HR 0.62; 95 % CI [0.43–0.87]). Similar to the AATAC trial, all patients had AF recurrence monitored with an implanted device. A mortality benefit (13.4 % versus 25 %; HR 0.53; 95 % CI [0.32–0.86]; p=0.01) was also demonstrated in the ablation arm, which was driven by a lower rate of cardiovascular death.21
CASTLE-AF builds on the accumulating evidence that catheter ablation may have benefits in patients with HF but does not necessarily add clarity as to which patients with HF should be targeted for ablation. The patients appeared to be highly selected with >3000 screened for eligibility, but only 13.2 % ultimately enrolled. Patients whose implanted device was from a different vendor (study sponsored by Biotronik) were excluded (32.4 %). Among the ablation arm, 69 % had NYHA class I or II HF, 40 % had ischaemic cardiomyopathy and 30 % had paroxysmal AF. Long-standing persistent AF was observed in 28 % of patients in the ablation arm and 30 % in the medical therapy arm. This clearly reveals a highly heterogeneous cohort of patients with HF. There was also significant heterogeneity in terms of the catheter ablation procedure itself with the aim of the procedure to isolate all pulmonary veins and achieve sinus rhythm. Additional lesions involving the cavo-tricuspid isthmus, roof, superior vena cava and inferior vena cava were permitted at the discretion of the operator.
Although promising, the results of CASTLE-AF may not apply to asymptomatic patients with HF, older patients (with a median age of 64 years in the study) as well as patients with advanced HF. Indeed, there is some evidence to suggest that some groups of patients with HF respond much better to catheter ablation. In the Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction (CAMERA-MRI) study, patients with idiopathic cardiomyopathy and persistent AF were randomised to undergo either catheter ablation or medical rate control.22 All patients had a cardiac MRI scan at baseline after optimisation of rate control to assess LVEF and presence of late gadolinium enhancement (LGE) – a surrogate of ventricular fibrosis. These patients were younger (mean age 59 ± 11 years in ablation arm and 62 ± 9.4 years in the medical therapy arm) and had a mean LVEF of 35 %, but a large majority (72–76 %) had long-standing persistent AF. The primary outcome was a change in LVEF during a repeat MRI at 6 months. The ablation group had a better improvement in LVEF compared with medical rate control, but, more interestingly, among patients undergoing catheter ablation, those who were LGE negative at baseline had an even better response compared with those who had evidence of LGE on MRI. These findings indicate that restoration of sinus rhythm in a cohort of patients with no other apparent cause of their cardiomyopathy may result in improved LV function, but a pure effect of improved heart rate cannot be excluded for the effect seen.
The true value of CAMERA-MRI may be that risk stratification tools such as cardiac MRI with LGE could identify a cohort of patients who may be super-responders to catheter ablation.22 These findings are consistent with a previous report by the same authors in which 15/16 LGE-negative patients with long-standing persistent AF who underwent catheter ablation maintained sinus rhythm at 6 months with a significant improvement in LV function.23 However, it remains unclear if ablation improves outcomes beyond LV function in the CAMERA-MRI study as there were no data on HF-related hospitalisations or mortality in this cohort.
Complication rates related to the catheter ablation procedure may also be higher in patients with HF compared with general cohorts of patients undergoing an AF ablation, which bears consideration during patient selection. In the CASTLE-AF study, procedure-related complications or serious adverse events occurred in 7.8 %, while in a contemporary cohort of general patients undergoing AF ablation the complication rate was 2.3 %.24
Differences in Electrophysiological Substrate Within Patients with Heart Failure and Between Patients With and Without Heart Failure: Implications for Ablation Strategy
Persistent AF appears to be more prevalent than paroxysmal AF in patients with HFrEF.25 There is building momentum towards increasing the single-procedure success rates for paroxysmal AF with more reproducible, standardised PVI workflows, incorporating composite ablation indices such as ‘ablation index’ or tracking inter-lesion distance to minimise gaps between adjacent lesions.26,27 However, standardised approaches for catheter ablation of persistent AF remain a long way off, with the results of the Substrate and Trigger Ablation for Reduction of Atrial Fibrillation Trial Part II (STAR AF II) demonstrating no benefit of additional linear ablation or ablation of complex fractionated activity (CFE) to PVI alone in this population.28 This is particularly relevant in patients with HF as the results from an international multicentre registry have suggested that long-term success rates for persistent AF ablation are significantly lower in patients with HF compared with those without HF (57.3 % versus 75.8 %; p<0.001), while there was no significant difference in success rates for paroxysmal AF ablation (78.7 % versus 85.7 %; p=0.186).29
Most of the clinical trials of AF ablation in HF described above have had significant heterogeneity between studies in terms of the ablation protocol used, while data comparing different ablation strategies in HF populations are sparse. In a meta-regression analysis of clinical trials and observational studies of AF ablation in HF patients, there was no difference in sinus rhythm maintenance between a PVI approach versus extensive left atrial ablation (linear lesions or CFE ablation).30 However, there are some cardiomyopathies with extensive left atrial structural remodelling such as hypertrophic cardiomyopathy or cardiomyopathies secondary to valvular heart disease.12 A higher prevalence of CFE has also been reported in some groups of patients with HF.12 Only a minority of these patients with significant structural and electrical remodelling have undergone PVI alone in observational studies.12 Whether PVI alone is adequate or sufficient or whether more aggressive substrate modification strategies are required in these groups of patients remains unclear.
Even in patients with paroxysmal AF, those patients with HFrEF appear to have more non-PV triggers than patients with normal LVEF. Furthermore, in a cohort with HFrEF, when ablation of non-PV triggers was performed in addition to PVI, a significantly improved long-term ablation success was achieved compared with PVI alone (75.0 % versus 32.2 %; p<0.001).31
There are important structural and anatomical abnormalities in the atria of patients with HF compared with patients without HF that may impact on their electrophysiological properties. Using a cohort of patients with symptomatic HF and age-matched controls, Sanders et al. demonstrated that patients with HF had an increase in atrial effective refractory period, no change in the heterogeneity of refractoriness and an increase in atrial conduction time along the low lateral right atrium and coronary sinus.32 They also found evidence of functional delay at the crista terminalis and indirect evidence of conduction slowing across the left atrium and Bachmann’s bundle.32 Taken together, these electrophysiological differences may have led to the observation of increased inducibility and duration of AF in patients with HF.32 Specific causes of cardiomyopathy such as valvular heart disease, which may cause advanced structural remodelling in the left atrium, also demonstrate different conduction patterns. Patients with persistent AF associated with mitral regurgitation (MR) had greater conduction delay and anisotropy in the posterior left atrium associated with fractionated electrograms in these regions compared with patients with MR but without AF.33
The ideal AF ablation protocol in patients with HF remains unclear. Although PVI alone may be appropriate in certain groups of patients, further investigation and innovation in ablation tools are required to clarify the role of additional linear lesions and develop reproducible strategies in patients with HF and persistent AF. In cohorts of high-risk patients with advanced structural remodelling, extensive left atrial ablation may be needed first-line, but an individualised strategy with a greater understanding of the underlying electrophysiological substrate will likely be required.
Interestingly, in a recent study, Halder et al. used CFEs as a surrogate marker of substrate complexity.34 In patients without HF who had persistent AF, a higher baseline burden of CFEs was seen in both the left and right atrium compared with patients with AF and HF. This finding challenges the belief that the presence of structural heart disease leads to additional complexity in atrial substrate. However, the importance of distinguishing between which is the initiating disease may be relevant to explain these findings – whether AF occurred first leading to subsequent development of HF or whether HF occurred first. Halder et al. suggest that those patients without HF with persistent AF may have a more complex primary bi-atrial substrate as a result of the primary electrical disturbance.34 In their study, a left atrial step-wise ablation strategy with more extensive substrate modification resulted in a higher single-procedure arrhythmia-free survival rate at 12 months in the HF group compared with the non-HF group.34 Taken one step further, whether identification of patients who develop AF first prior to HF development may be a means of stratifying which patients may respond to rhythm control with ablation remains unclear and warrants further investigation. In reality, this is difficult to perform in clinical practice due to the large numbers of patients presenting with both AF and HF with no clear indication of which was the primary disturbance.
Risk Stratification Tools for Atrial Fibrillation Ablation
Improved risk stratification tools to identify patients with AF and HF who might respond best to catheter ablation will be of great value to electrophysiologists to reduce unnecessary procedures in patients unlikely to benefit or to offer more procedures to patients most likely to see clinical benefit. A number of clinical parameters have been associated with clinical outcome following AF ablation including type of AF, age, gender, LVEF, left atrial size/volume, and presence of hypertension, obstructive sleep apnoea or diabetes.35
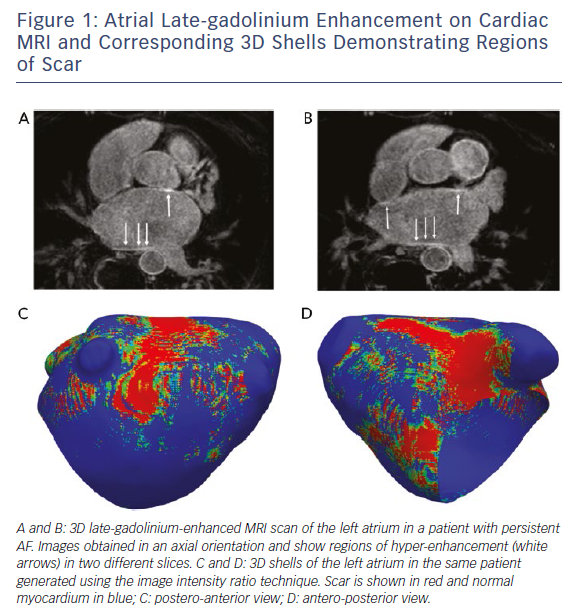
The use of cardiac MRI to assess left atrial (LA) structure and function has also led to the identification of additional parameters such as LA reservoir function,35 LA sphericity36 and LA fibrosis37 that may be related to ablation outcome. The extent of baseline atrial LGE (as a surrogate of fibrosis) (Figure 1), in particular, has received significant attention, with some centres reporting limited value in risk stratification,38 but a growing volume of literature supporting the belief that patients with a higher burden of atrial LGE have worse outcomes following AF ablation.39,40
Improved risk stratification tools to identify subsets of patients with HF who might respond better to catheter ablation are warranted and may also help to tailor ablation strategies. Based on the results of the CAMERA-MRI study, cardiac imaging, in particular, bears consideration in selecting the right patients for catheter ablation. The ongoing Delayed Enhancement MRI-guided Ablation Versus Conventional Catheter Ablation of Atrial Fibrillation (DECAAF-II) trial (NCT20529319) will examine the impact of targeting LGE-MRI detected atrial fibrosis during AF ablation to improve procedural outcomes. Although this study will include a general cohort of patients, it is likely that it will also include a subset with co-existing HF.
Non-invasive electrocardiographic imaging, whereby a subject undergoes a multi-detector CT scan while wearing a 252-electrode vest on the thorax to record epicardial unipolar electrograms to reconstruct epicardial electrical potentials on patient-specific geometry, also offers potential as a tool to guide ablation strategy.41 In patients with persistent AF, electrocardiographic imaging has been used to demonstrate the increasing complexity of AF drivers with prolonged AF duration.42 Prior knowledge of the principle locations of AF drivers may help guide ablation strategy in specific groups of patients.43
Based on results of completed clinical trials of AF ablation in HF (Table 1), patients who tend to have the least benefit from catheter ablation appear to have a higher NYHA functional class, longer duration of AF and extensive structural remodelling. Those who appear to respond best to catheter ablation have no other structural abnormalities related to their cardiomyopathy.22 There may be a third group of patients that have both AF and an underlying occult cardiomyopathy that persists despite improvements in AF burden after ablation.44
What is clear from all the trials to date is that HF populations with AF are highly heterogeneous; this can have a significant impact on clinical outcomes. However, the presence of significant structural heart disease does not universally imply more complex electrophysiological substrate and tailored strategies will likely be required to obtain the best clinical outcome.34
Future Perspectives
Despite significant progress in catheter ablation in patients with HF, a number of unanswered questions remain including the optimal means of risk stratification of patients with HF to AF ablation, optimal ablation technique and timing of catheter ablation. Whether intervention will be cost effective if patients require multiple re-do ablations, particularly as HF progresses, is also unclear. A number of clinical trials are currently underway that may provide some clarification. The Ablation of Atrial Fibrillation in Heart Failure Patients (CONTRA-HF) trial will investigate the impact of cryoablation in patients with HF and implanted cardiac devices/cardiac resynchronisation therapies (NCT03062241). An improved reproducibility of the ablation procedure itself is expected in the cryoablation arm and its impact on hard endpoints such as mortality will be welcome. The Catheter Ablation Versus Anti-arrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial will also assess the impact of ablation on the hard endpoints of mortality, stroke and hospitalisations in a large cohort (>2,000) of patients (NCT00911508). Patients with both HFrEF and HFpEF will be included in the study, which will likely give further insights into the optimal management of patients with AF and HF.
The Catheter Ablation Versus Medical Therapy in Congested Hearts with AF (CATCH-AF) trial will assess the impact of catheter ablation in patients with newly diagnosed symptomatic AF with the aim of assisting electrophysiologists in understanding the benefits of early AF ablation (NCT02686749). The Atrial Fibrillation Management in Congestive Heart Failure With Ablation (AMICA) trial will investigate whether PVI alone in patients with persistent AF or long-standing persistent AF improves outcomes compared with best medical therapy (NCT00652522). This will allow the impact of a standardised procedure to be investigated in a less heterogeneous group of patients.
The Randomised Ablation-based Atrial Fibrillation Rhythm Control Trial in Patients with Heart Failure and High Burden Atrial Fibrillation (RAFT-AF) will assess the cost-effectiveness of an ablation strategy in patients with HF as well as assess hard endpoints including all-cause mortality; patients with HF will be stratified according to those with HFrEF and HFpEF (NCT01420393).
The advent of permanent His bundle pacing to preserve physiological conduction of the ventricles and enable a means of achieving cardiac resynchronisation therapy could also offer an alternative management strategy in selected groups of patients with AF and HF, if combined with AV node ablation.45 Further studies to assess clinical outcomes using this strategy are anticipated.
Conclusions
AF ablation in certain patients with HF may be safe and effective, but most data in this setting are derived from experienced centres. Ablation may not be appropriate in patients with advanced HF, poor functional status or in those with extensive structural remodelling. Improved risk stratification tools and standardisation of ablation strategies in different groups of patients should lead to the development of patient-orientated approaches that seek to identify the patients most likely to benefit from catheter ablation and improve procedural success rates in those patients.







