Determining optimal treatment strategies for complex arrhythmogenesis in AF is confounded by the lack of consensus on the mechanisms causing AF. Fundamental to defining arrhythmogenic mechanisms of AF are the distinctions and interplay between functional features (determined by the electrophysiology of a cell) and structural features (determined by whether a structural or anatomical feature is critical to the existence and location of a source), as well as between hierarchical and anarchical mechanisms (determined by whether an arrhythmia is perpetuated by discrete drivers or a universally distributed random phenomenon, respectively). Current discussions focus on whether myocardial activation in AF exhibits any organisation and, if it does, whether this organisation is due to functional or structural properties of the tissue. The hierarchical theory of AF proposes a degree of organisation in AF, sustained by discrete electrical drivers, whereas the anarchical theory proposes that AF is sustained by a large number of randomly propagating, self-perpetuating activation wavelets without the presence of discrete electrical drivers.1–3 Differences in reported AF mechanisms may be because AF is recorded across diverse models, investigational tools, spatial scales and clinical populations, ranging from paroxysmal to permanent AF.
With this motivation, what follows is a series of definitions of the key mechanistic phenomena and classifications. This article outlines the proposed potential mechanisms of AF, describes the different data modalities and analysis techniques used, indicates the challenges associated with interpretation of AF mechanisms and how these may be overcome and suggests areas of future research. Throughout this review, possible explanations for divergent findings between studies are suggested.
Mechanisms of AF
Here we briefly describe some of the concepts that are proposed to underlie AF, which is defined as a high-frequency turbulent electrical activity in the atria. Sustained AF requires the presence of both a driver initiating the arrhythmia (consisting of either impulse initiation by automaticity or triggered activity, or re-entrant activity) and a substrate that causes fibrillatory conduction. AF mechanisms depend on the degree of electrical and structural remodelling, which changes as AF progresses from paroxysmal to persistent to permanent AF. This is described in detail in the review by Schotten et al.1
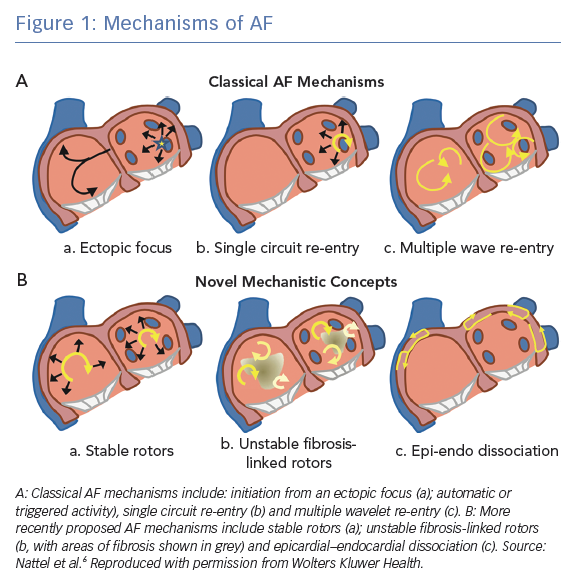
Proposed AF mechanisms include automaticity and triggered activity, both of which are examples of abnormal impulse formation, as well as re-entrant mechanisms. Both automaticity and triggered activity may initiate re-entry and manifest as waves emanating centrifugally from a focal source. Although a single focus of automaticity is likely to be too slow to drive AF, recurrent triggered activity may maintain AF by continuously causing fibrillatory activity in the atria.4 During paroxysmal AF, these electrical triggers and ectopic beats are frequently located in the pulmonary veins.5 In this review, we focus on re-entrant mechanisms, where ‘re-entry’ is defined as the repetitive excitation of tissue by a recirculating wavefronts. Figure 1 shows several of the proposed mechanisms involved in the initiation and maintenance of AF. These mechanisms include the classical AF mechanisms of a single ectopic focus, single circuit re-entry and multiple wavelet re-entry, as well as more recent mechanistic concepts of stable rotors, unstable fibrosis-linked rotors and epicardial–endocardial dissociation.6
Arrhythmia initiation and maintenance, by mechanisms including re-entry, depends on the arrhythmia substrate, which we define as the electrophysiological and structural properties that underlie arrhythmia initiation and maintenance. Features of this substrate may be anatomical or functional.
Anatomical Re-entry
Anatomical re-entry occurs when a wavefront of excitation propagates around an anatomical obstacle and re-excites myocardium that it has previously excited to form a re-entrant circuit. Following on from Mayer’s experiments in 1906,7 Mines suggested a model of fixed anatomical re-entry in 1913 based on experiments in atrial and ventricular ring-like preparations that could be responsible for tachyarrhythmias in humans.8 Mines showed that re-entry around such a circuit required the product of the wave conduction velocity and refractory period (the wavelength) to be smaller than the length of the circuit (the path length). For example, macro re-entry around cardiac structures, such as the tricuspid annulus (a cause of atrial flutter), occurs when this condition is satisfied, and the length of the path and the conduction velocity determine the cycle length of the activity.9
Anatomical re-entry may also occur at the micro scale with the movement of a wavefront around a small anatomical obstacle such as a small region of fibrosis sustaining fibrillatory conduction. As such, micro-anatomical obstacles anchor re-entrant wavefronts; Tanaka et al. demonstrated that fibrosis in heart failure (HF) determines AF dynamics as re-entrant sources anchor to areas of fibrosis in Langendorff-perfused HF sheep atria.10 Hansen et al. used late gadolinium enhanced (LGE) MRI and dual optical mapping to show that re-entrant drivers anchor to micro-anatomical tracks maintaining AF.11,12
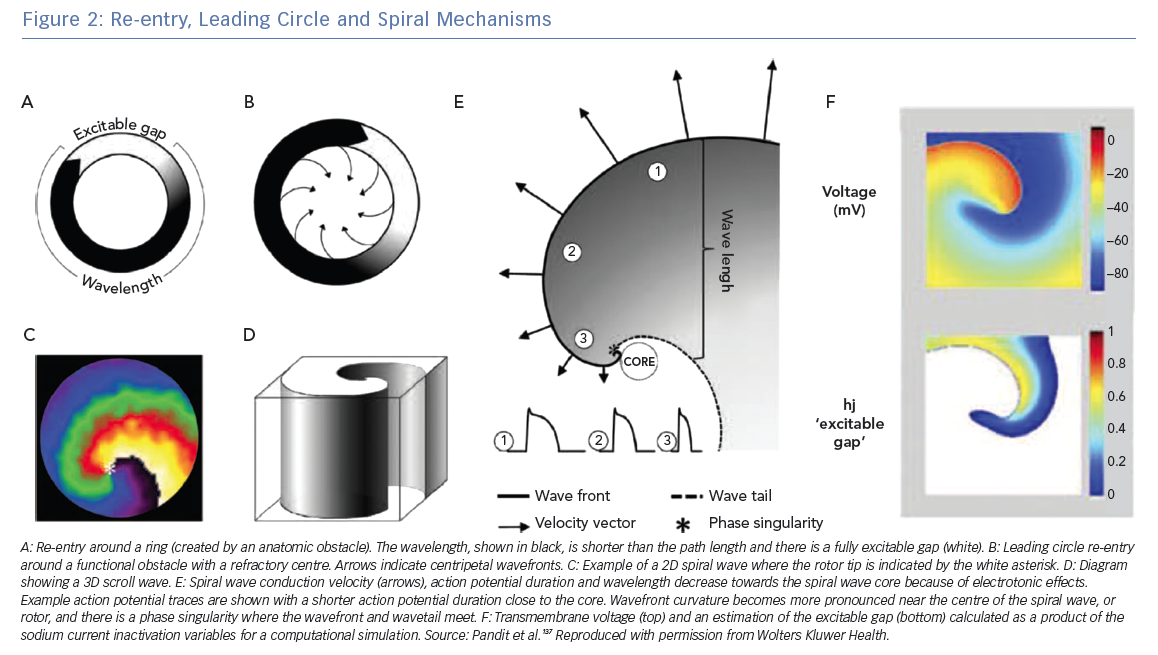
All re-entrant circuits, whether anatomical or functional (see below), must have an excitable gap, which is the short time interval during the re-entry cycle when excitation by an external impulse is possible. This gap can be partially or fully excitable. Anatomical circuits can have a partially excitable gap when the wavelength just fits the path length or a fully excitable gap when the wavelength is significantly shorter than the path length. Figure 2A shows a schematic re-entry with a fully excitable gap.
Functional Re-entry
Following on from Garrey’s suggestion in 1914 that re-entry could be initiated without an anatomic obstacle, in 1973 Allessie et al. provided the first direct experimental evidence that the presence of an anatomical obstacle is not necessary for re-entry, demonstrating the existence of functional re-entry.13,14 We define functional re-entry as re-entrant activity in the absence of a predetermined anatomical obstacle or circuit. Functional conduction block occurs when cardiac activation fails due to source–sink mismatch.
Leading Circle Mechanism
In 1973, Allesie et al. proposed the ‘leading circle’ theory in which a unidirectional block (due to a heterogeneous distribution of refractory period) causes an excitation wavefront to travel in a circular pathway.14 In this theory, wavefronts also travel centripetally (towards the centre of the circle) and centrifugally (away from the centre). This theory is called ‘leading circle’ because there is a main circle that takes the path corresponding to the smallest possible circuit for which the path length equals the wavelength (approximately equal to the conduction velocity multiplied by the effective refractory period); centripetal wavefronts travelling over shorter circuits hit refractory tissue, whereas centrifugal wavefronts are dominated by the faster rate of the leading circle (Figure 2B).15
The leading circle theory does not have a fully excitable gap but must have a partially excitable gap. The leading circle wavefront travels through partially refractory tissue, which reduces the conduction velocity, in turn reducing the wavelength.16 The central area is refractory because it is stimulated twice as fast as the leading circle activation by the centripetal wavefronts, leading to an unexcitable region. The inclusion of centripetal wavefronts in this model was motivated by the presence of low-amplitude, short-duration deflections; however, this observation is also compatible with contemporary spiral wave theory. The leading circle theory does not take into account the role of wavefront curvature, which is a very important component of rotors and the spiral wave mechanism.
Spiral Wave Theory
Spiral waves are ubiquitous in nature and excitable media; for example, spiral waves occur in chemical reactions (e.g. the Belousov–Zhabotinsky reaction), morphogenesis of amoeba,17 mitochondrial calcium waves in frog eggs18 and chicken retina.19 Spiral wave theory for cardiac arrhythmias was developed in theoretical studies performed by Krinsky in the USSR in the 1960s and by Winfree in the US.20,21 The first experimental evidence for the existence of spiral waves in cardiac tissue was from Davidenko et al. in sheep ventricular muscle.22 A rotor is a classification of functional re-entry where wavefront curvature is the cause of the wavelength being shorter than the path length. The wave of excitation emitted by the rotor is a spiral wave in two dimensions or a scroll wave in three dimensions.23 Figure 2C shows a spiral wave and Figure 2D shows a schematic scroll wave. The convex curvature of the wavefront increases towards and attains a critical value at the centre, and conduction velocity slows such that the wavefront cannot propagate into the core. The decrease in conduction velocity, action potential duration and wavelength due to electrotonic effects is illustrated in Figure 2e. At the centre, the wavefront curvature is so high that the wavefront source cannot provide enough current to depolarise the resting sink tissue ahead of it, causing rotation. As such, this core area is excitable but not excited, in contrast with the full refractory centre of the leading circle theory. The centre of rotation, or core, is the organising centre of the spiral or scroll wave.
The activation and repolarisation wavefronts meet each other at a non-excited point known as a phase singularity (PS), at which the phase of activation is undefined, and all excitation–recovery phases converge. Figure 2E shows the PS point where the wavefront and wave tail meet. A stationary rotor will have a PS that follows a circular trajectory, whereas meandering rotors have more complex trajectories. The trajectory of the PS path determines the diameter of the spiral wave core. The spiral wave theory has no fixed wavelength; wavelength also likely changes in the leading circle model as the re-entrant wavefront moves from transverse to longitudinal conduction in anisotropic tissue.
The mother rotor hypothesis proposes that AF is not entirely random, but that hierarchical periodic rotors drive the AF, acting as sources of high-frequency wavefronts.24 The leading circle theory and spiral wave theory are different models to explain functional re-entry. One of the key differences between the models is that they predict different responses to sodium channel blockade, with the leading circle theory predicting that re-entry is promoted by reducing the wavelength and the spiral wave theory predicting an antiarrhythmic action because of increased meander, increased core size and decreased critical curvature, which is consistent with experimental findings.15 In addition, the leading circle theory does not explain the observation that wavelength is not reduced in several experimental models and many AF patients.25
Kléber and Rudy state that a freely rotating wavefront in an excitation–diffusion system has to be spiral shaped because velocity must decrease from the edge to the centre of the wave to satisfy a constant period of rotation and because the velocity of a convex wavefront is less than that of the linear wavefront at the edge.16 As such, leading circle theory was a historically considered mechanism, whereas spiral wave theory is a useful contemporary concept.
Wavefronts from a mother rotor may break into multiple wavefronts: wavebreak occurs when a wavefront encounters an obstacle (e.g. scar tissue), leading to the formation of daughter wavefronts, or wavelets, and fibrillatory conduction.
Many studies report that AF re-entrant circuits are unstable13,26,27 and of short duration,28,29 which challenges the theory that discrete drivers sustain AF. An emerging novel hypothesis to explain how unstable re-entrant circuits may sustain AF is the idea of continuous phase singularity regeneration or ‘renewal’, which was initially proposed by Dharmaprani et al.30
Multiple Wavelets
The multiple wavelet hypothesis, initially proposed by Moe and Abildskov in 1959, states that AF is a disorganised anarchical atrial rhythm in which there are multiple random activation wavelets sustaining the activity, independent of the initiating event.31 Moe et al. developed a computational model and predicted that at least 26 wavelets are required to sustain the arrhythmia.32 Experimental support for this hypothesis came from the Allessie group, who found that between four and six wavelets were required to sustain turbulent atrial arrhythmia with the application of acetylcholine to dog hearts.33 However, the multiple wavelet hypothesis does not explain the origin of the activity that causes the wavelets; if there were a small number of wavelets, then one would expect them to coalescence and annihilate AF.24
The Cox–Maze surgical procedure aims to terminate AF by using surgical incisions to reduce the atrial tissue mass below the critical circuit size required by multiple wavelet re-entry.34 In addition, computational modelling studies have investigated potential approaches for ablating multiple wavelet activation. For example, Carrick et al. simulated different ablation lesion sets to test the effects of ablation lesion length and multiple wavelet circuit density on ablation outcome, finding that applying ablation at regions of high circuit density most efficiently decreased re-entry duration.35 Carrick et al. then extended this to predict the most efficient distribution of ablation lesions for multiple wavelet activation.36
Breakthrough Activation
Allessie et al. found no evidence for the presence of stable focal sources or rotors in a human epicardial mapping study using a high-resolution mapping catheter (interelectrode distance 2.25 mm) during cardiac surgery.37 Instead, they proposed a novel theory for the development of AF in structural heart disease, where the endocardium and epicardium of the atrium become electrically dissociated and epicardial breakthrough leads to fibrillatory waves.38 Electrical activation arising on the endocardial or epicardial cardiac tissue surface from transmural propagation through the cardiac tissue is termed breakthrough activation. This breakthrough could be focal from the other surface of the heart or due to transmural re-entry. This represents a limitation of the endo–epicardial dissociation theory because it is difficult to determine whether it is a unique mechanism or a manifestation of transmural scroll waves.21 Although how best to treat an AF substrate with endocardial–epicardial dissociation is an open question, recent studies by Jiang et al. and Piorkowski et al. demonstrate the feasibility of AF catheter ablation based on epicardial and endocardial substrate mapping.39–41
Classification of Mechanisms
Different studies group together different functional and anatomical mechanisms for the presentation and interpretation of their findings. For example, Weiss et al. classify the leading circle and spiral wave theories as functional re-entrant mechanisms, separate from anatomical re-entry even when the rotor is anchored by an anatomical (fibrous tissue) core.4 Richter et al. differentiate between anatomically anchored spiral waves and functional spiral waves, which may meander.42 In contrast, Nattel et al. do not make this distinction and consider that the spiral wave theory also explains rotors anchored to anatomical obstacles, effectively considering all re-entrant mechanisms together.43 A rotor that is anchored to an anatomical obstacle that is large enough to become its centre of rotation cannot be distinguished from anatomical re-entry. Similarly, Krogh-Madsen et al. classify re-entry in their model as a mother rotor, even though it is anchored.44
We suggest dividing mechanisms into abnormal impulse initiation and abnormal impulse conduction, following Hoffman and Rosen.45 Using this classification, re-entry is then a general subheading under abnormal impulse conduction that includes anatomical and functional re-entry. Importantly, a rotor does not require an anatomical obstacle according to its definition; adding an obstacle will anchor a rotor but is not a necessary component of its mechanisms. Conversely, micro re-entry around an anatomical obstacle need not be a rotor.
Cardiac Mapping Techniques
Some of the divergence in mechanisms observed across studies may be due to the different analytical techniques used; as such, we review commonly used methodologies here.
Activation Time Mapping
Charting local activation time from extracellular recordings (electrograms) on anatomical maps (electroanatomical mapping) are key to determining mechanisms of atrial flutters, tachycardias and slower regular rhythms because they indicate the pattern of activation, including electrical circuits and focal sources. However, activation time mapping for AF data is much more challenging because fractionation in the electrogram signals makes activation time assignment difficult, signals change continuously over time and it is difficult to select a suitable time window in which to display these maps. The local activation time of a unipolar electrogram is defined as the time of the maximum downslope because this has been shown to correspond to the time of maximum upstroke of the action potential and maximum sodium conductance, providing a biophysical basis for this choice of marker.46 In contrast, the choice of marker for the activation time of bipolar electrograms does not have a biophysical basis and varies between studies, with choices including the maximum absolute amplitude and the maximum derivative.47 Unipolar electrograms represent a more local signal, but are often contaminated by artefacts from the ventricles; bipolar electrograms typically eliminate the ventricular signal, but their amplitude depends on wavefront direction.48,49
The Schotten laboratory developed a technique to automatically assign activation times and reconstruct wavefronts from unipolar AF data.50 Activation time mapping analysis groups together similar local activation times into fibrillation waves. Activation time maps can be post-processed to calculate conduction velocity maps.51–53
Electrogram Features
Techniques to analyse fibrillatory electrogram data include frequency analysis, such as dominant frequency or organisational index calculations, fractionation scoring analysis, continuous electrical activity calculation, gradient of activation calculation, Shannon entropy analysis and peak-to-peak voltage calculation.54–59
Features of the electrogram indicating properties of the underlying atrial structure may be identified and targeted during ablation with the aim of eliminating electrical drivers. Clinical mapping studies have used different measures to target electrical drivers, including identifying sites of high dominant frequency (DF; the frequency with the highest power in the power spectrum obtained by applying the fast Fourier transform). DF analysis may be performed on invasive or non-invasive recordings; for example, Guillem et al. identified sites of maximal DF from non-invasive body surface potential mapping data.60 Areas of high DF are thought to indicate areas of driver activity, and some clinical studies have targeted these areas.61 Sanders et al. demonstrated that ablating areas of high DF prolonged AF cycle length and increased AF termination for paroxysmal but not persistent AF.54
In advanced forms of AF, areas of slow activity are also important, and targeting areas of high DF is unlikely to provide sufficient ablation therapy. Jarman et al. found that areas of high DF are not spatiotemporally stable, suggesting that they do not represent a fixed driver.62 Salinet et al. suggest instead targeting areas that are repeatedly of high DF.63 Shariat et al. propose using regional DF analysis to identify regions of wavebreak.64 The Radiofrequency Ablation of Drivers of Atrial Fibrillation (RADAR-AF) trial showed that ablating high-frequency sources, identified using DF analysis, together with pulmonary vein isolation (PVI) is not significantly different to using PVI alone.65 This highlights that the usefulness of DF for targeting ablation is questionable because the arrhythmia mechanism is unstable.66,67 A key technical challenge for frequency mapping that needs to be taken into consideration is that the temporal resolution is limited by the short duration of cardiac recordings compared with the sampling rate.
Nademanee et al. proposed that fragmented electrograms represent areas where AF is perpetuated.55 Ablation of complex fractionated atrial electrograms (CFAE) terminated AF in 95% of patients in their study.53 However, other groups have failed to replicate this success.68,69 One confounding factor is that there are different definitions of fractionated electrograms, with the clinically used electroanatomical mapping software using different algorithms to calculate CFAE scores, which have been shown to correlate poorly with each other and with conduction velocity and the number of waves per AF cycle.70
In addition, it is difficult to separate the mechanisms underlying electrogram morphology. Narayan et al. mapped local refractoriness of atrial tissue using monophasic action potential (MAP) catheters to classify the fractionation of bipolar electrograms, finding that far field signals account for 67% of fractionation and that other CFAE types include rapid localised AF sites (8%), spatial disorganisation (17%) and CFAE following AF acceleration, which is often accompanied by MAP alternans (8%).71 A high-density mapping study of patients during AF, sinus rhythm and paced rhythms showed that CFAE distribution is highly variable and often caused by wave collision.72
Electroanatomical mapping data may be processed to calculate the peak-to-peak amplitude of each bipolar electrogram signal across the atrium to construct a spatial map of voltage. Areas of low voltage may identify regions of fibrotic tissue. Marcus et al. investigated the spatial distribution of voltage, demonstrating that AF patients exhibit more low-voltage areas on the septal and posterior walls.59 Jadidi et al. combined PVI ablation with ablation guided by electrogram voltage to show improved outcomes for persistent AF compared with PVI alone.73 Box isolation of fibrotic areas is an ablation approach that applies patient-specific lesions surrounding areas of low-voltage tissue.74 One challenge associated with voltage mapping of bipolar electrogram signals is that the amplitude of bipolar electrogram signals depends on wavefront direction. Omnipolar mapping technology has the potential to overcome this limitation by providing an orientation-independent measure of voltage.75
Phase Mapping
Despite fibrillation being a seemingly random process, Gray et al. developed a technique to analyse fibrillatory signals to translate periodicity in the signals into loops in a two-variable-state space that represents the system.76 For phase mapping, the two-variable system consists of the signal at a particular location plotted against a time-delayed version of the signal. The phase angle is then measured as the angle around this trajectory for each point in the domain, and a spatial singularity in phase then corresponds to the centre of a rotating wave.76 The landmark paper of Gray et al. revealed a degree of spatiotemporal organisation in fibrillation, and the technique used to reveal this organisation is one method that can be used to locate the tip of spiral waves and analyse their dynamics. More recently, the Hilbert transform has been used to create a time-delayed signal, and techniques have been developed for phase mapping of unipolar and bipolar electrogram data.77,78 Topological rules enforce that the ends of wavefronts must be connected either to each other, to boundaries or to phase singularities.79
Phase mapping has been used by several clinical centres to guide ablation therapy. For example, the focal impulse and rotor modulation (FIRM) software applies phase mapping to basket electrode catheters to identify electrical drivers as ablation targets.80 Non-invasive ECG imaging (ECGi) technologies consist of a vest of body surface electrodes for electrical recordings together with an imaging scan to provide anatomical information, with these being combined to construct detailed electroanatomical maps.81 Phase mapping has been applied to ECGi recordings to identify the spatiotemporal distribution of electrical drivers during AF, with ablation focused on the high-density regions.81 Recent clinical review papers provide more details on electrical driver determination in AF.82,83
Activation Versus Phase Mapping
A potential advantage of phase mapping over activation mapping is that phase mapping does not assign particular importance to an activation point, which is advantageous for fractionated signals in which it is difficult to assign an activation time. Methodologies for constructing phase maps consist of both pre- and post-processing algorithms. Preprocessing steps may be used to construct sinusoidal signals from atrial recordings prior to the application of the Hilbert transform to calculate phase. For example, Kuklik et al. developed a sinusoidal recomposition technique for unipolar electrograms in which an electrogram signal is expressed as a sum of sinusoidal wavelets of one period length.78 Although this technique does not explicitly require activation times to be assigned to the signal, it assumes a constant cycle length for the signal to define the sinusoidal wavelets.
Kuklik et al. compared cycle lengths calculated from times assigned to the unipolar signals to those calculated from the times of phase inversions and showed a good correlation.78 Roney et al. developed a technique for phase mapping of unipolar or bipolar electrograms that uses a sequence of filters and a variation of a pseudoempirical mode decomposition technique to preprocess the signals prior to phase analysis.77 Filtering the electrogram signals removes high-frequency components of the signal, which may represent activation for fractionated signals.
Post-processing steps include interpolation and extrapolation of activation time recordings or phase values measured at a sparse arrangement of points either to a regular grid or to the entire atrial surface. We previously demonstrated that the spatial resolution of AF data can significantly affect the interpretation of the underlying AF mechanism,84 which is a particularly important consideration when interpreting findings from low-resolution recording devices.85 Jacquemet investigated the effects of different phase interpolation techniques on false-positive and -negative phase singularity detections.86
Clinically, both activation time and phase mapping techniques are challenging to apply to sequentially acquired AF recordings due to the temporal instability of AF. For globally acquired data, activation mapping is feasible but challenging due to electrogram fractionation and because it requires the choice of a time window in which to display activation wavefronts. Phase mapping has been successfully used to guide clinical ablation approaches,81 but requires specialist analysis techniques. It is important to ensure that differences in findings between clinical centres are not because of differences in analysis techniques. Consequently, we recommend applying multiple analysis techniques to the same electrical dataset to increase confidence in the findings; for example, using both activation and phase mapping or using alternative phase mapping techniques.3,87
Fibrosis Mapping
Previous studies have demonstrated an association between AF driver location and fibrosis distribution: rotors are observed at the borders of patchy scar in clinical non-invasive ECGi studies88 and in modelling studies.89 As such, fibrotic areas represent an alternative target for catheter ablation. One of the challenges associated with clinical implementation of atrial LGE imaging is the requirement for standardised image processing techniques and, as such, Sim et al. published a standardised, reproducible open-source platform for AF assessment.90 Areas of fibrosis may be identified as areas of low voltage and ablated (box isolation of fibrotic areas74) or imaging data may be used to identify areas of high LGE intensity. Kircher et al. compared applying PVI together with either linear ablation or ablation of low-voltage areas to find that ablating low-voltage areas increased arrhythmia-free survival rate.91
The Delayed-Enhancement MRI (DE-MRI) Determinant of Successful Radiofrequency Catheter Ablation of Atrial Fibrillation (DECAAF) study showed that atrial fibrosis detected on LGE-MRI was independently associated with AF recurrence.92 However, other studies found no correlation between LGE and rotors.93 Efficacy of Delayed Enhancement MRI-Guided Ablation vs Conventional Catheter Ablation of Atrial Fibrillation (DECAAFII; NCT02529319) is a current clinical study investigating whether ablation guided by LGE-MRI is superior to PVI.94 Chen et al. compared identifying arrhythmogenic areas as sites with spatiotemporal dispersion or continuous activity to low-voltage areas and areas of increased intensity on LGE-MRI to find that most arrhythmogenic activities colocalised with low-voltage areas, but there was less colocalisation with fibrosis identified using LGE-MRI.95 Modelling studies may aim to select regions of fibrosis most likely to harbour re-entrant drivers.96
Data Challenges
Data Modality
For catheter ablation cases, different clinical centres use different catheters and electroanatomical mapping systems, each of which has its own advantages and disadvantages, which must be considered in data interpretation.
Contact Mapping Systems
Multiple high-density electrode plaques have been used to map the epicardial atrial surface during surgery. For example, de Groot et al. used a spoon-shaped device with 244 unipolar electrodes (diameter 3.6 cm; interelectrode distance 2.25 mm), as well as a rectangular array of 8 × 8 electrodes (interelectrode distance 2.5 mm) to demonstrate the presence of focal fibrillation waves due to epicardial breakthrough.38 In addition, Lee et al. collected simultaneous data from three epicardial electrode arrays with a total of 510–512 electrodes (total area 92.85 cm2) and showed that wavefronts from foci or breakthrough maintained AF, with no evidence of re-entry.26
High-density mapping catheters offer high-fidelity signals at good spatial resolution (2–6 mm), but are limited in their coverage (diameter 2–3.5 cm), and so data have to be collected sequentially to construct a global map. These electrogram recordings may be processed to construct global maps of electrogram features, including DF values and fractionation indices. Both the Biosense Webster Carto and the Abbott EnSite Precision electroanatomical mapping systems offer toolboxes to assess electrogram fractionation using different algorithms,70 which may inform ablation strategies. Constructing activation maps from AF data in which activation patterns may be complex and continuously changing is challenging. To address these challenges, Mann et al. developed an algorithm called RETRO-Mapping to detect wavefront propagation from sequential AF recordings.97
The Rhythmia system (Boston Scientific) has been used with the Orion mini-basket catheter (Boston Scientific) to map atrial tachycardia to identify entrance and exit gaps at high resolution.98 High-density catheters can be used to identify missed pulmonary vein–atrial connections after pulmonary vein ablation.
Recently, an omnipolar mapping technology, which provides orientation-independent measurements of cardiac activation and voltage, has been developed and integrated in the Abbott EnSite Precision electroanatomical mapping system.99 The system uses a high-density grid of 16 equidistant electrodes (HD Grid Mapping Catheter Sensor Enabled; Abbott Technologies), with 3-3-3-mm spacing to provide improved localisation of scar, lesion gaps and wavefront collision.100 Hong et al. used this catheter for mapping of the atria to differentiate between far- and near-field signals and to assess bidirectional conduction block after PVI.101
Basket catheters record endocardial electrograms and offer a more global coverage; however, this coverage is limited to the atrial body and reduced by bunching of splines. For example, Laughner et al. measured interspline distances in the LA ranging from 1.5 to 121.2 mm, with one-third of mapping electrodes exhibiting poor contact.85 FIRM is a clinical mapping system that uses a basket catheter and phase mapping technology to identify rotors and focal sources in patients undergoing ablation for AF.102 Using the technology revealed that AF was sustained by an average of two to three rotors or focal sources, within a mean (± s.d.) area of 2.2 ± 1.4 cm2, which were then ablated.103 The technology has shown an improved clinical outcome compared with conventional ablation in many studies; however, a recent study showed that catheter ablation of sites identified by FIRM mapping terminated AF in only a minority of patients.104 The CARTOFINDER software (Biosense Webster) within Carto may be used with basket mapping catheters to identify rotational and focal activation areas.105
Non-contact Mapping Systems
Non-contact electrode mapping systems, such as the dipole density mapping AcQMap system (Acutus Medical), which is used together with ultrasound imaging, offer a global coverage at a high resolution. The Utilizing Novel Dipole Density Capabilities to Objectively Visualize the Etiology of Rhythms in Atrial Fibrillation (UNCOVER-AF) 127-patient trial used AcQMap technology together with other ablation technologies to show promising results for freedom from AF at 1 year.106
Body surface ECGi mapping has the advantage that it reconstructs signals from the epicardium of most of the left and right atria; however, it may not map the atrial septum and the pulmonary veins, and signals are smoothed during the inverse calculation. In addition, the technology does not map the endocardium.81
Endocardial Versus Epicardial Surface Recordings
The choice of endocardial or epicardial mapping will affect recordings and may explain differences between, for example, findings from ECGi and basket mapping studies. For instance, the electrical activity on the endocardium and epicardium of the atrium during AF has been shown to exhibit degrees of discordance, in which there are periods where the surfaces show the same wavefront pattern and times when they have different wavefront patterns.107 Hansen et al. found that intramural drivers were seen on subendocardial optical mapping, but these manifested as either re-entry or breakthrough patterns on subepicardial mapping.11
Differences Between Studies
Recent clinical studies have published disparate findings on the mechanisms underlying AF. For example, the Signal Transfer of Atrial Fibrillation Data to Guide Human Treatment (STARLIGHT) clinical trial found no evidence of sustained rotational drivers; instead, persistent AF in these patients was sustained by multiple wavelets of activation.3 Navara et al. demonstrated the existence of rotational and focal activation in pulmonary vein antral regions for cases in which ablation terminated AF before complete PVI.87 Honarbakhsh et al. used the CARTOFINDER technology together with a basket catheter to identify transient but repetitive focal or rotational drivers.108
Ablation Approaches
AF ablation approaches differ in their anatomical or electrical targets, as well as in the methodologies and recording devices used to identify these targets. PVI remains the cornerstone of AF ablation, and ablation approaches for persistent AF typically include PVI together with other ablation lesions. Ablation approaches may target features of the electrogram signal; for example, Nademanee et al. pioneered the ablation of CFAE signals, demonstrating a high success rate.55 However, other clinical centres using CFAE ablation failed to replicate these outcomes, possibly due to the different aetiologies of fractionation.72 An alternative ablation approach is to target areas of high frequency identified using DF analysis. However, the RADAR-AF trial showed that ablating high-frequency sources, identified using DF analysis, together with PVI was not significantly different to using PVI alone.65 Ablation techniques that target specific electrogram features, including the degree of fractionation, spatiotemporal dispersion57 or areas of DF, have the advantage that they can be applied to sequentially acquired recordings, from readily available catheters. The Substrate and Trigger Ablation for Reduction of Atrial Fibrillation Trial – Star AF II Study (Star AF II) found no improvement in ablation outcome with the addition of linear ablation or CFAE ablation to PVI for persistent AF patients.109
Globally acquired recordings may be post-processed using phase mapping to identify electrical drivers that are targeted during ablation. This approach demonstrated promising success rates using basket catheters in the Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation (CONFIRM) trial, but other clinical centres showed varied outcomes using the technique.102,103 Phase mapping has also been applied to non-invasive ECGi recordings to identify and target electrical drivers during AF.81 These techniques require recording devices with global coverage and specialist analytical techniques.
Alternatively, some ablation approaches target areas of fibrotic remodelling. These may be identified as regions of low voltage using sequential electrical mapping and isolated electrically using box isolation of fibrotic areas74 or imaging techniques; for example, DECAAFII is a clinical study investigating whether ablation guided by LGE-MRI is superior to PVI.92 Ablation approaches may also aim to modify the electrical size of the atria or target specific anatomical structures.110,111
Spatial and Temporal Resolution
Recording modalities are typically limited in either resolution or coverage, as explained in the previous section. We investigated how spatial resolution affects interpretation of AF recordings, expressing spatial resolution requirements as a linear function of the spatial wavelength, and found that high-density multipolar catheters provide sufficient resolution for rotor and focal source detection, but that the basket catheter is prone to false rotor detections.84 Aronis and Ashikaga considered the effects of multiple coexisting rotors on resolution requirements and found that including more than one rotor increased errors 10-fold, suggesting higher resolution requirements for cases with multiple drivers.112
Data Processing
Correct processing of unipolar electrograms requires careful QRS subtraction.113 Spatial interpolation of voltage will create problems if electrograms have different degrees of contact, and bipolar amplitude is direction dependent. Interpolation of phase does not have these problems; however, phase must be interpolated as a circular variable.84,86 Pathik et al. analysed basket catheter electrograms and reported 2D rotors that are not present in 3D, suggesting that correctly incorporating distance between splines in 2D analysis is important.114 Reliable detection of activation times for atrial electrograms during AF is challenging, particularly for fractionated signals.
Differentiating Between Mechanisms Using Limited Data and Interpolation
Phase mapping including data interpolation will not be able to differentiate between a leading circle and spiral way mechanism because both will appear as a spiral wave with a phase singularity after analysis.
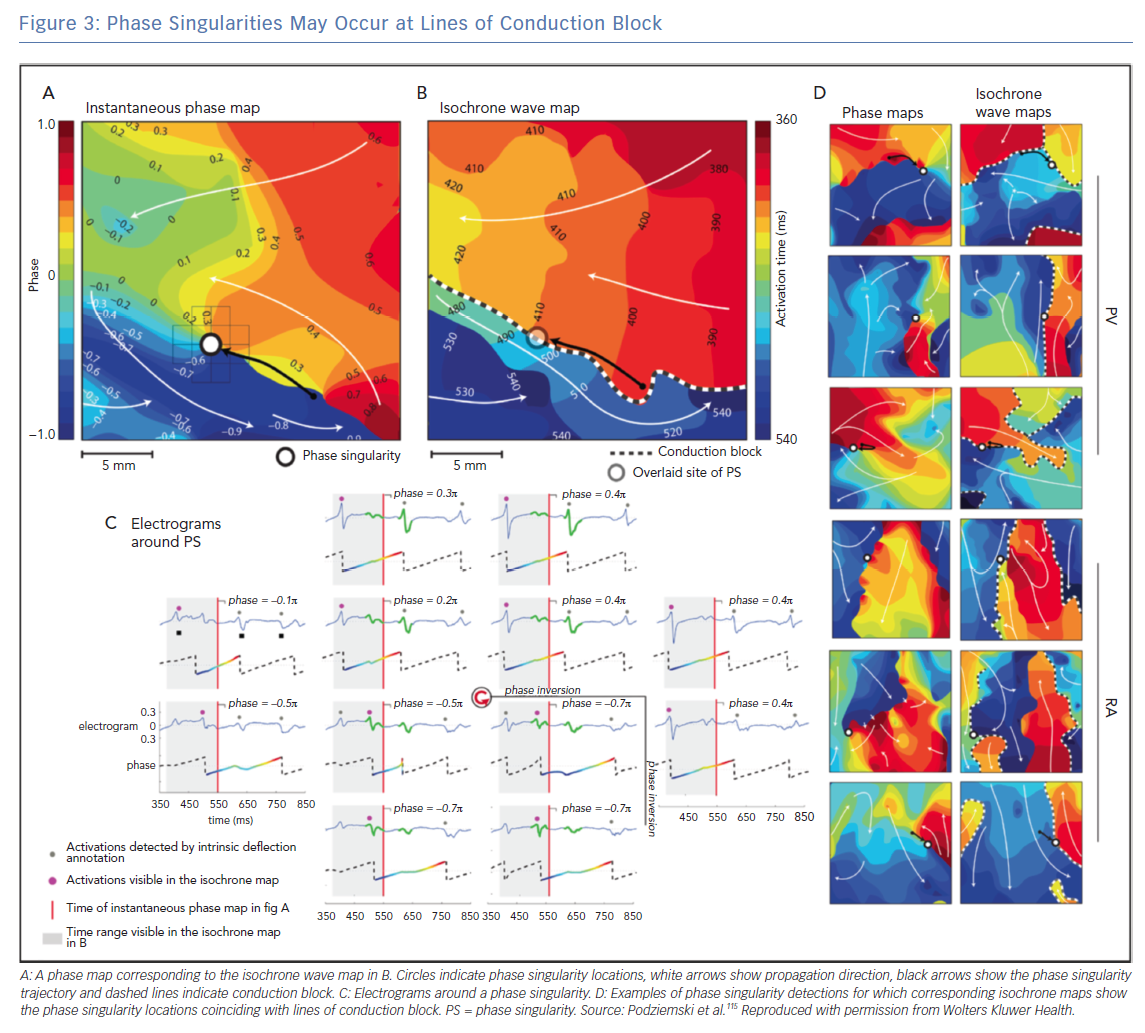
The interpretation of phase mapping of conduction block requires particular care. Podziemski et al. demonstrated that analysis of conduction block data may result in phase singularities that are not due to rotational wavefronts;115 an example is shown in Figure 3 in which phase singularity locations coincide with lines of conduction block. Spiral waves with linear cores have been observed in both computational and experimental studies (core size 1–2 cm), which may appear similar to conduction along a line of block. Topologically, wavefronts must end on either a boundary or PS, so there will be a PS at the end of a wavefront moving along a line of block. Considering the rate of change of phase around a PS point, or the magnitude of conduction delay, may indicate whether the PS is at a fixed rotor core or a conduction block line (which may be a linear core). In addition, using computational simulations, Martínez-Mateu et al. showed that far-field components of unipolar electrograms make it difficult to distinguish between functional and anatomical re-entry.116 An alternative interpretation of the findings of Podiziemski et al. is motivated by the work of Arthur Winfree, who states that rotors are seldom symmetric;21 the core of a rotor is often elongated because of the anisotropic properties of conduction (the long axis of the ellipse would be in the longitudinal direction of fast conduction) and described as an arc of functional conduction block.
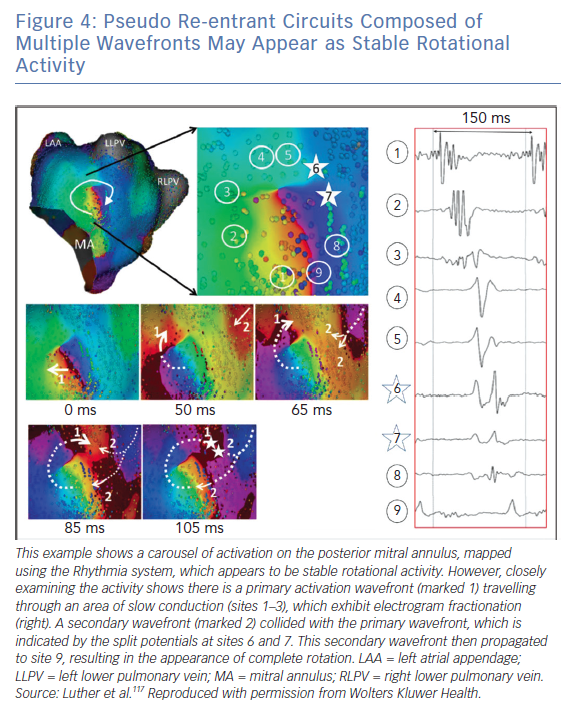
Luther et al. investigated re-entry during atrial tachycardia using the Rhythmia system and showed that pseudo re-entrant circuits often appear as stable rotational activation.117 This is shown in Figure 4, in which a secondary wavefront colliding with a partial rotational circuit gives the appearance of a complete rotational circuit.
To correctly interpret arrhythmia mechanisms and to determine appropriate ablation approaches, it is important to differentiate between stable rotational activation and pseudo re-entrant circuits. This distinction is important for determining how ablation lines affect individual wavefronts during arrhythmia. During AF there will be more wavefront collisions and conduction around lines of block, making correct interpretation even more complex.
Future Perspectives
There are differences in opinion over how to classify re-entrant mechanisms, for example whether leading circle and spiral re-entries should be classified separately and whether a re-entry anchored to a small structural obstacle should be considered an anatomical re-entry or a functional spiral wave. We recommend following Hoffman and Rosen, dividing mechanisms into abnormal impulse initiation and abnormal impulse conduction.45 Re-entry is then a general subheading under abnormal impulse conduction that includes anatomical and functional re-entry, with anatomical re-entry around a central anatomical obstacle. A rotor does not require an obstacle according to its definition; adding an obstacle will anchor a rotor but is not a necessary component of its mechanisms. Conversely, micro re-entry around an anatomical obstacle need not be a rotor.
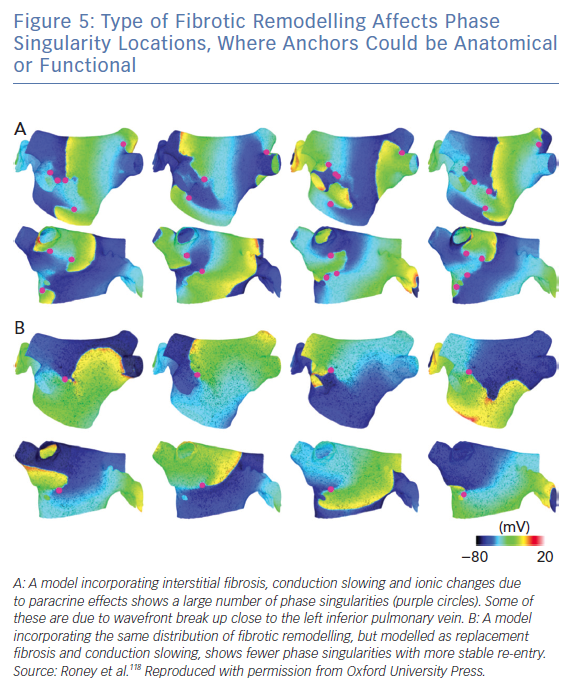
Interestingly, anchors caused by fibrotic remodelling could be anatomical (including micro-anatomical re-entry caused by insulating collagen) or functional, due to the action potential duration and conduction velocity properties of tissue in the presence of fibrosis (Figure 5).118 An ablation line from the centre of the re-entrant circuit to a boundary of the tissue theoretically works for both anatomical and functional cases. As the same ablation approach may work in either case and we cannot differentiate between these mechanisms clinically, considering these mechanisms as hierarchical, as opposed to anarchical, could be a beneficial classification.
Ensuring the correct classification of phase singularities may prove crucial in their use for targeting ablations because wavefront break up does not represent an equal target to a stable rotor. Targeting regions of the atria with a high probability of drivers may be a promising ablation strategy in the instance that drivers are an important AF mechanism. Increased understanding of the reason for this is warranted, including the development of methodologies for determining the relative importance of different drivers in the case of multiple drivers.119 Perhaps, uncovering a degree of order in anarchical AF paves the way for the identification of ablation targets. Thus, future studies into anarchical AF, to investigate whether any order exists, are paramount.
Ablation strategies for AF either target anatomical structures, use information on the structural substrate from imaging data or use information on the electrical substrate from electroanatomical mapping. For example, Pambrun et al. systematically targeted the coronary sinus and the vein of Marshall, the pulmonary veins and any anatomical isthmus block regions, showing that this lesion set provides good short-term outcomes.111 The DECAAFII clinical trial ablation strategy is to isolate areas of fibrotic tissue identified using LGE-MRI.92 Recent ablation approaches using electroanatomical mapping data include the stochastic trajectory analysis of ranked signals (STAR) mapping approach, which identified early sites of activation and ablated these to produce a favourable clinical outcome.120 Future research directions include how best to combine anatomical, structural and electrical measures to guide ablation therapy and to assess the additional benefit of mapping AF to provide patient-specific ablation approaches.
Understanding the tissue properties underlying AF is important for designing treatments aimed at limiting disease progression. Further studies linking the atrial substrate and arrhythmia, similar to that of Zhao et al., will advance the mechanistic understanding of AF and its ablation.12 The degree of re-entrant driver meander may be decreased by both anatomical and electrophysiological properties (e.g. by application of acetylcholine). Re-entry anchor location and driver formation may also depend on electrophysiology, conduction velocity dynamics, cardiac wavelength and anisotropy.52,121–124 These tissue and electrophysiological properties each affect the electrogram signal, but inferring these individual properties from the electrogram signal is challenging.
Simultaneous optical and electrical mapping will enable increased understanding of the relationship between electrogram and transmembrane voltage features.125 In addition, detailed cellular-level mapping of the electrical properties of the centre of re-entrant activity, extending the study of Houston et al., will enable identification of arrhythmia mechanisms and will bridge the cellular and tissue levels.126
Further clinical, basic science and computational studies investigating optimal ablation approaches for these different arrhythmia mechanisms are required. For example, Bayer et al. used computational modelling studies to suggest an alternative ablation approach that aims to streamline activation patterns.127 Roney et al. performed a virtual pilot clinical study to use simulations to predict whether an extreme ablation approach of ablating interatrial connections would return the right atrium to sinus rhythm.128 In addition, Weiss et al. examined the effects of ablation lesions on mother rotor activity, showing that ablation at the core may convert the functional re-entry to a slower anatomical re-entry, whereas ablating from the core to a border interrupts the circuit and terminates the arrhythmia.4 Finally, we recommend the design of new mapping catheters based on the resolution, data type and analysis methods discussed here.
Computational models of atrial arrhythmia have been used to offer important insights into arrhythmia mechanisms.128–130 A recent pioneering study from the Trayanova laboratory identified patient-specific targets for AF for patients with a fibrotic substrate.131 However, patient-specific modelling of AF is challenging due to the anatomical and structural complexity of the atria and the dynamic nature of the electrical substrate. Future research into improved methodologies for model construction, calibration and uncertainty quantification is required for aspects including segmentation,90 anatomical structures,128 electrical and structural anisotropy,52,132,133 repolarisation heterogeneity and restitution,134 conduction heterogeneity,51 registration,135 fibrotic remodelling118 and performing predictions on clinical timescales.136
Identifying the properties of the atrial substrate responsible for sustaining the arrhythmia (e.g. critical areas of fibrosis) may potentially be important for understanding the arrhythmia. Electrical mapping results need to be interpreted carefully, alongside other measures of the substrate, to identify the sustaining mechanisms of the arrhythmia.
Clinical Perspective
- AF mechanisms include anatomical and functional re-entry, hierarchical drivers that include re-entry and triggered activity, and anarchical multiple wavelets.
- Data challenges, including differences between recording devices in spatial and temporal resolutions, spatial coverage and recording surface, may account for differences in reported AF mechanisms.
- Identifying the properties of the atrial substrate responsible for sustaining an arrhythmia (e.g. critical areas of fibrosis) may potentially be important for understanding the arrhythmia.
- Electrical mapping results need to be interpreted carefully, alongside other measures of the substrate, to identify the sustaining mechanisms of the arrhythmia.