In patients with syncope or episodes of palpitations and heart disease, an electrophysiology study (EPS) may be of value by means of potential induction of sustained ventricular tachyarrhythmias.1,2 Programmed ventricular stimulation may be useful in the context of risk stratification of ischaemic patients with left ventricular ejection fraction (LVEF) 30–40 %, and there has been some evidence that it might also be of predictive value in revascularised patients with ST-segment elevation myocardial infarction and LVEF ≤30 %.3,4 In patients investigated for bradycardias, either due to sinus node or atrioventricular (AV) conduction disturbances, the role of EPS is not well defined. Most of the time, the criteria used for indications of permanent pacing are symptoms and electrocardiographic findings. However, there are certain occasions on which an EPS is mandatory either for the establishment of diagnosis or for appropriate implementation of prophylactic pacing.
Sinus Bradycardia
Early studies on limited patient cohorts had suggested that corrected sinus node recovery time (c-SNRT) may be useful in predicting the development of syncope and the need of permanent pacing in patients with bradycardia. A marked prolongation of the c-SNRT and an absent or blunted response to atropine and exercise suggest impaired sinus node function.5 However, a wide range of ‘normal’ c-SNRTs has been published, and it seems that only very prolonged c-SNRTs (>800 ms) have reasonable predictive ability.6 When considering conventional upper limits, such as 500–550 ms, the sensitivity of the test in asymptomatic patients with dizziness or no symptoms who will need a pacemaker in the future is only 50–65 %.7 The sinoatrial conduction time (SACT) is an insensitive indicator, being prolonged in only 40 % of our patients with clinical findings of sinus node dysfunction.5 The ACCF/AHA/HRS 2012 Guidelines on Device-based Therapy recommend EP testing in patients with syncope of unexplained origin for the detection of clinically significant abnormalities of sinus node function (IIa-C indication for permanent pacing),8 whereas the ESC 2013 Guidelines on Pacing and Cardiac Resynchronization Pacing do not consider the value of EPS established in this setting.9
AV Conduction Disturbances
Diagnosis
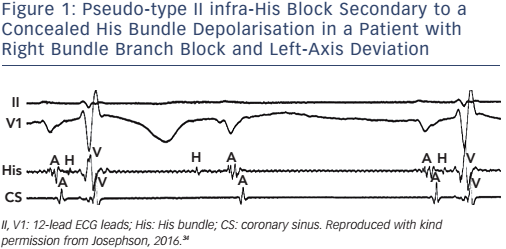
The ECG appearance of first- or second-degree block may be due to junctional (His bundle) extrasystoles that are concealed (not conducted to the atria or ventricles), but render a portion of the conduction system refractory to propagation of a sinus beat. The observance of junctional premature depolarisations on the surface ECG suggests that concealed His bundle extrasystoles are responsible for the apparent A-V block, but a His-bundle recording is the only method of positive identification (see Figure 1).
Anatomic Site of Block
High-grade block can occur anywhere in the AV conduction system, and the width of the QRS complex and the configuration of conducted beats and/or escape beats are of only limited value in localising the site of block. A narrow QRS complex is most compatible with an AV nodal or intra-His problem, and a wide QRS complex is most compatible with an infra-His problem; however, a wide QRS complex certainly may occur with A-V nodal or intra-His disease in the presence of coexistent bundle branch block.10 Approximately 70 % of type II blocks (i.e. consecutive, non-conducted P waves without changes in the PR interval) are associated with bundle branch block, whereas 30 % are associated with a narrow QRS complex, and are therefore within the His bundle.11 All type II blocks are infranodal, i.e. below the AV node or N zone, but not all infranodal blocks are type II blocks.12 Although 2:1 or higher degrees of block (e.g. 3:1 and 4:1) have traditionally been classified as type II block, the site of those blocks cannot be reliably determined by the surface ECG. A 2:1 block in the context of bundle branch block does not necessarily indicate infranodal block, since in 15–20 % of patients the block is in the AV node. Thus pacing may not be required in asymptomatic patients with this pattern and an EPS may be useful (see Table 1). Symptomatic patients with type I block and a bundle branch block should also be considered for an EPS to determine the site of block. Multiple levels of A-V block spontaneously or during pacing may also coexist in the same patient, and they can produce a confusing ECG picture that is extraordinarily difficult to interpret without an EPS. Combined A-V nodal and infra-His block has been described as the mechanism in alternate 2:1 block and prolonged PR intervals.13 Finally, the presence of phase 4 block can be suspected by observing prolonged ventricular asystole and the absence of type I block in long tracings. Absence of sinus slowing is usually a criterion for type II block because a vagal surge can cause simultaneous sinus slowing and AV nodal block, which can superficially resemble type II block. Significant PR prolongation before and after block and prolonged P-P intervals during ventricular asystole are indicative of vagal block that is a benign condition, rather than paroxysmal AV block, i.e. pause-dependent phase 4 AV block which is potentially dangerous for syncope.14 A definitive diagnosis of phase 4 AV block require His bundle recordings.11
Specific loss-of-function SCN5A mutations demonstrate varied competing gating effects that reduce sodium current density and enhance slow inactivation with selective slowing of conduction velocity, in a way that may result in isolated progressive conduction system disease without provoking tachyarrhythmias.15 Initial EPS in mutation carriers with AV block revealed prolonged HV but normal A-H intervals, thus suggesting infra-Hisian block, but progressive AV block due to SCN5A mutations can be both supra- and infra-Hisian. Thus, an EPS may useful in this setting. Several different types of muscular dystrophies, such as Emery-Dreifuss muscular dystrophy, limb girdle muscular dystrophy (Erb’s muscular dystrophy), myotonic dystrophy type 1 (Steinert’s disease) and desmin-related myopathy, are also associated with progressive conduction defects. An EPS may identify patients in need of permanent pacing, and improve survival of these patients.16
Prediction of High-Grade AV Block
H-V Interval
Bifascicular block, specifically right bundle branch block (RBBB) with left-axis deviation, is the most common ECG pattern preceding complete heart block in adults.5 Early studies on patients with bifascicular or trifascicular block have reported an overall incidence of progression to complete heart block of approximately 1–2 % per year.17–20 Patients with syncope, in particular, may have a 17 % cumulative incidence of complete AV block within the next 5 years.19 The evaluation of the patient with bundle branch block or fascicular block necessarily involves testing the integrity of the remaining fascicle, and the simplest method of assessing this His–Purkinje reserve is the measurement of basal H-V intervals (normal <55 msec). In the presence of bundle branch block, with or without additional fascicular block, the H-V interval should be normal as long as conduction is unimpaired in the remaining fascicle. Most patients developing complete infra-His block have prolonged H-V intervals (>70 ms),18–20 and prolongation of the HV interval by ≥13 ms is strongly associated with AV block following transcatheter aortic valve replacement.21 Thus, analysis of H-V interval was the factor initially evaluated as a predictor of subsequent heart block. However, an H-V interval >70 ms may not independently predict development of complete heart block,19 and approximately 50 % of patients with RBBB and left anterior hemiblock, and 75 % of patients with left bundle branch block (LBBB) have prolonged H-V intervals.22 This finding alone, therefore, is nonspecific as a predictor of the development of high-grade heart block, because the incidence of heart block is low, yet the presence of prolonged H-V interval is great. In our experience, approximately 70 % of patients with H-V intervals ≥100 msec, develop second- or third-degree infra-His block within the next two years. However, H-V intervals in excess of 100 msec are uncommon. Thus, such marked H-V prolongation, although highly predictive, is insensitive.

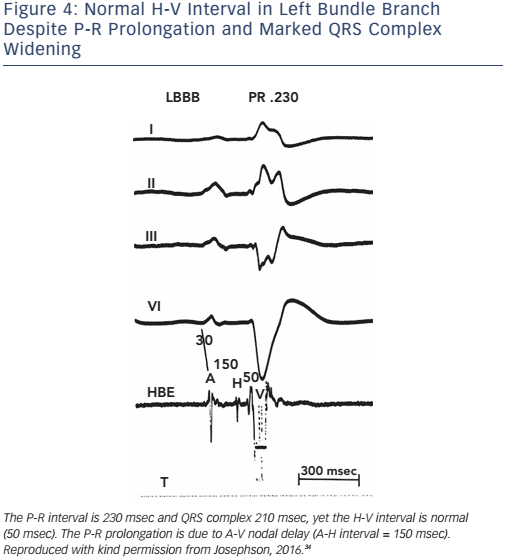
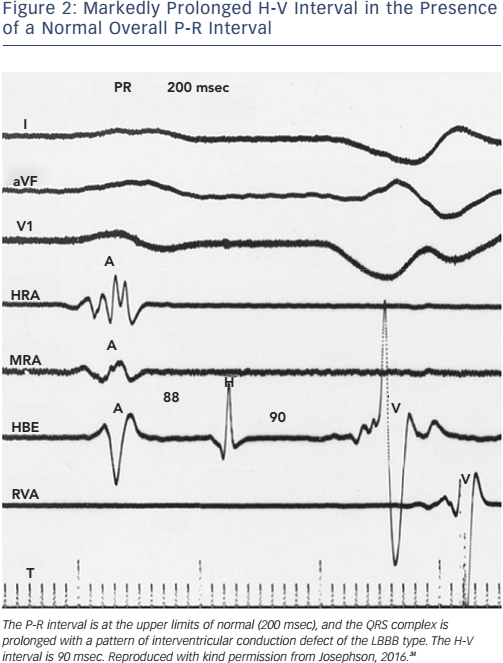
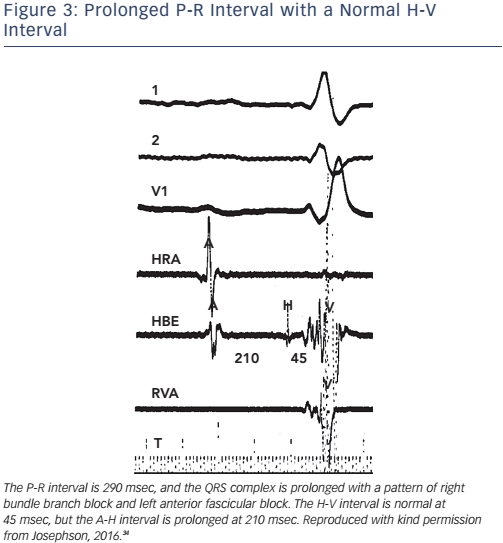
Of note, the surface ECG may not allow accurate assessment of the H-V interval. Although a short P-R interval (i.e. ≤160 msec) makes a markedly prolonged H-V interval (i.e. ≥100 msec) unlikely, and a P-R interval of >300 msec almost always means at least some AV nodal conduction abnormality,23 intermediate values do not correlate with the H-V interval (see Figure 2). In the presence of RBBB and left anterior hemiblock or LBBB with or without left anterior hemiblock, a normal P-R interval can easily ‘conceal’ a significantly prolonged H-V interval, and a prolonged P-R interval can be the result of a prolonged A-H interval only (see Figure 3). Most patients who have LBBB have prolonged H-V intervals, regardless of the length of the accompanying P-R interval. Conversely, a long P-R interval does not automatically mean a long H-V interval (see Figure 4).
Pharmacological Challenge
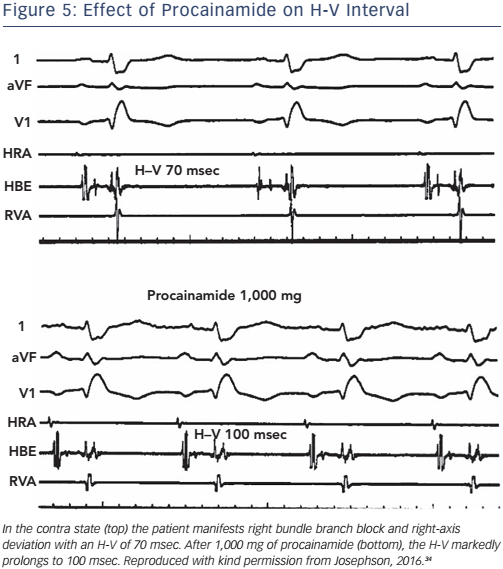
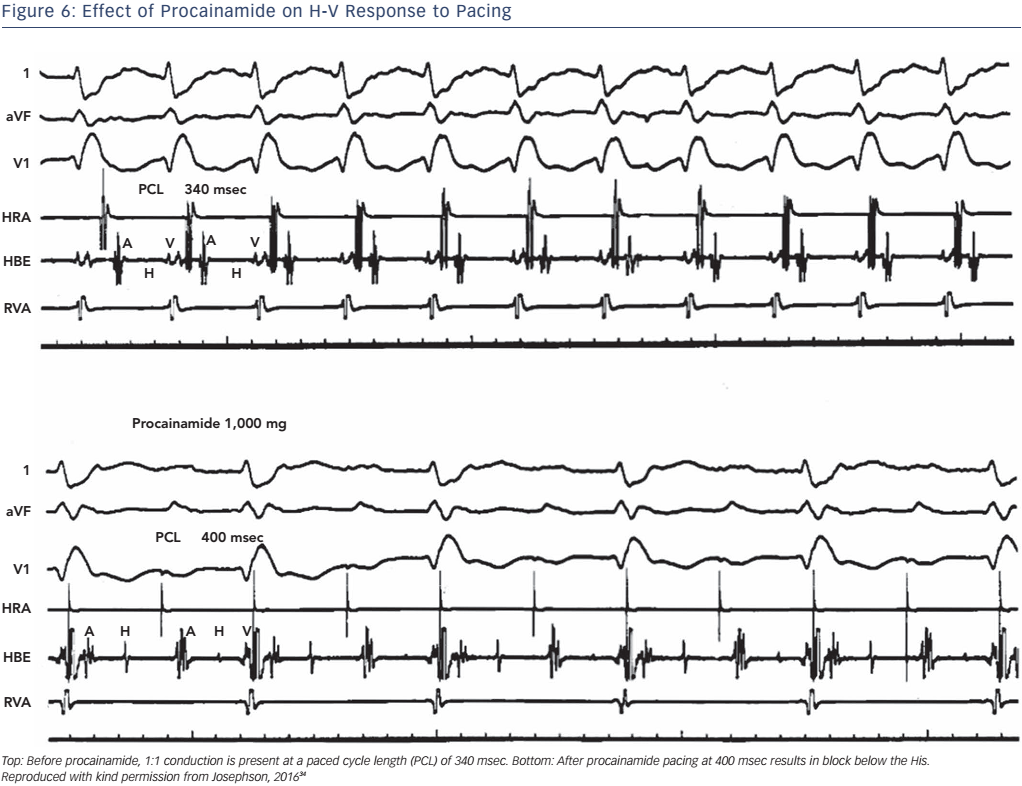
The administration of pharmacological agents known to impair His– Purkinje conduction (e.g. procainamide) may unmask poor His–Purkinje system reserve. In normal persons as well as in most persons with moderately prolonged (55–80 msec) H-V intervals, procainamide typically produces a 10–20 % increase in the H-V interval.24 An increase of greater magnitude, including (a) doubling of the H-V interval, (b) a resultant H-V interval exceeding 100 msec, or (c) the precipitation of second- or third-degree infra-His block, all represent evidence of propensity for spontaneous infra-His block (see Figures 5 and 6). However, the incidence of provocation of AV block by procainamide is low, and the test may be useful in cases with borderline prolongation of the HV interval but its diagnostic yield is rather limited.25–27
Other drugs may also be used in order to provide additional information about the His-Purkinje reserve. If the conduction improves with atropine or exercise, or worsens with carotid sinus massage, the block is in the AV node. If the conduction worsens with atropine or exercise or improves with carotid sinus massage, the block is in His or bundle branches.22 The value of adenosine or ATP testing for induction of asystole due to latent sinus nodal disease AV block in patients investigated for unexplained syncope is not established, and these drugs have little or no effect on the His-Purkinje system.28,29 However, there has been evidence that induction of cardiac pauses (due to AV block or sinoatrial block >10 sec by an IV bolus of 20 mg ATP) indicates the need for DDD pacing in patients with unexplained syncope.30,31 Adenosine may also be used although its effects are not identical to those of ATP.29,32
Atrial Pacing
The use of atrial pacing to stress the His–Purkinje system may provide further information beyond that of the basal H-V interval. Most normal patients will not exhibit second- or third-degree infra-His block at any time during incremental pacing, particularly at rates less than 150 bpm.
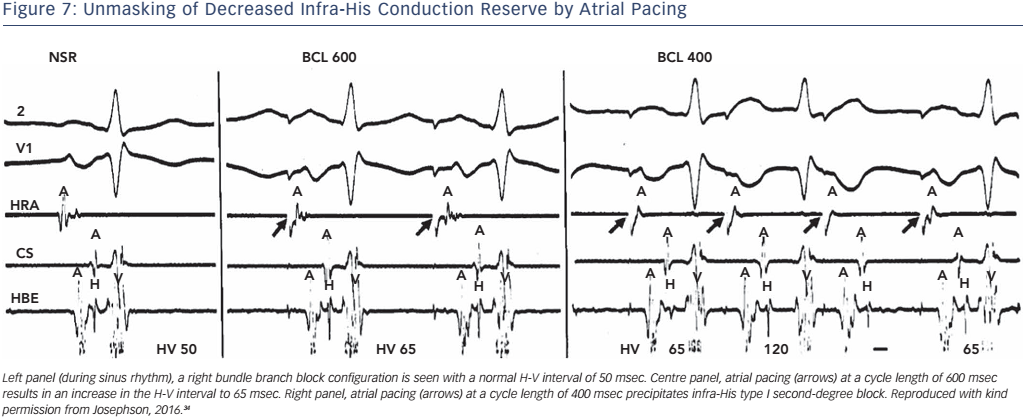
Physiologically, this occurs because the shortening of His–Purkinje refractoriness had decreased paced cycle lengths or because A-V nodal block developed at shorter paced cycle lengths, which thus protects the His–Purkinje system, even H-V prolongation during atrial pacing at rates less than 150 bpm. Second- or third-degree block within the His–Purkinje system in the absence of a changing A-H interval at paced cycle lengths of 400 msec or greater is abnormal and suggests a high risk for A-V block (see Figure 7). In patients who develop block distal to the His bundle induced by atrial pacing at rates of 150 bpm or less, there is a 33 % progression to high-grade A-V block within the next 3 years.33 However, data on this procedure, in order to guide the decision for permanent pacing, are limited.
Table 1 presents current recommendations of learned societies for the utility of EPS in the selection of patients with AV conduction disturbances for permanent pacing. In Table 2 we have summarised all indications of EPS for diagnostic purposes in patients with apparent or suspected AV conduction abnormalities.
Conclusion
In sinus bradycardia the role of EPS is not established. In AV conduction disturbances, there are certain occasions in which an EPS is necessary for the appropriate diagnosis, and allows both the avoidance of unnecessary permanent pacing and the appropriate implementation of prophylactic pacing.
Clinical Perspective
- The value of electrophysiology studies in patients with sinus bradycardia is not established. A marked prolongation of the corrected sinus node recovery time (>800 ms) is a highly predictive but insensitive sign for sick sinus syndrome.
- In patients with atrioventricular node conduction disturbances an electrophysiology study may be necessary to define the site of block.
- An H-V interval >70 ms is a nonspecific predictor of development of high-grade atrioventricular block. An H-V interval >100 ms is highly predictive but insensitive.
- Adenosine triphosphate/adenosine, procainamide and atrial pacing have a rather limited diagnostic yield in patients with AV conduction disturbances.