Coronavirus disease 2019 (COVID-19) is caused by the novel betacoronavirus officially named by the WHO as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It has spread rapidly globally since the first case reported in Wuhan, China in December 2019 and has now affected more than 12 million people worldwide, with varying fatality rates across different countries.1 The clinical presentation of COVID-19 is heterogeneous and varies from asymptomatic to severe pneumonia/acute respiratory distress syndrome (ARDS) requiring invasive mechanical ventilation. In addition, a hyperinflammatory state secondary to cytokine release syndrome leads to hypercoagulation, multiorgan failure and increased mortality.
COVID-19 has had a huge and unprecedented global impact on public health and healthcare delivery across several countries. Most electrophysiology (EP) activities have been significantly reduced or deferred in order to accommodate the healthcare demands of the pandemic. Irrespective of challenges faced during the pandemic, electrophysiologists still play a vital role in managing cardiovascular complications related to COVID-19 and in maintaining services for urgent and emergency EP procedures.
COVID-19 and the Cardiovascular System
The pathogenesis of COVID-19 is characterised by an initial phase of viral response followed by a host inflammatory response.2 During the early viral infection phase, the virus infiltrates the lung parenchyma and replicates. Collateral tissue injury and the inflammatory process that follows cause vasodilatation, endothelial permeability, and leucocyte recruitment leading to further pulmonary damage, hypoxaemia and cardiovascular stress.3,4 Several recent studies have demonstrated a deleterious impact on the cardiovascular system including acute myocardial injury, acute myocarditis, cardiomyopathies, arrhythmias, sudden cardiac death and cardiac arrest.5,6
Angiotensin-converting enzyme 2 (ACE2) acts as a host receptor, facilitating entry of the SARS-CoV-2 infection into human cells, and is expressed in lung alveolar epithelial, heart, vascular and gastrointestinal tract cells.7,8 Even though it is not certain at this time, ACE2 host receptor involvement may account for the clinical presentations with cardiovascular complications, such as myocarditis, arrhythmia and cardiogenic shock. This would also explain the trend of raised cardiac troponin T seen in these patients who are severely affected, and the correlation with disease severity and mortality.9,10 In several studies a significant proportion (22–33%) of patients with severe COVID-19 were found to have acute myocardial injury, as evidenced by raised cardiac troponin (above upper limits of normal) or new electrocardiographic/echocardiographic abnormalities.11–14
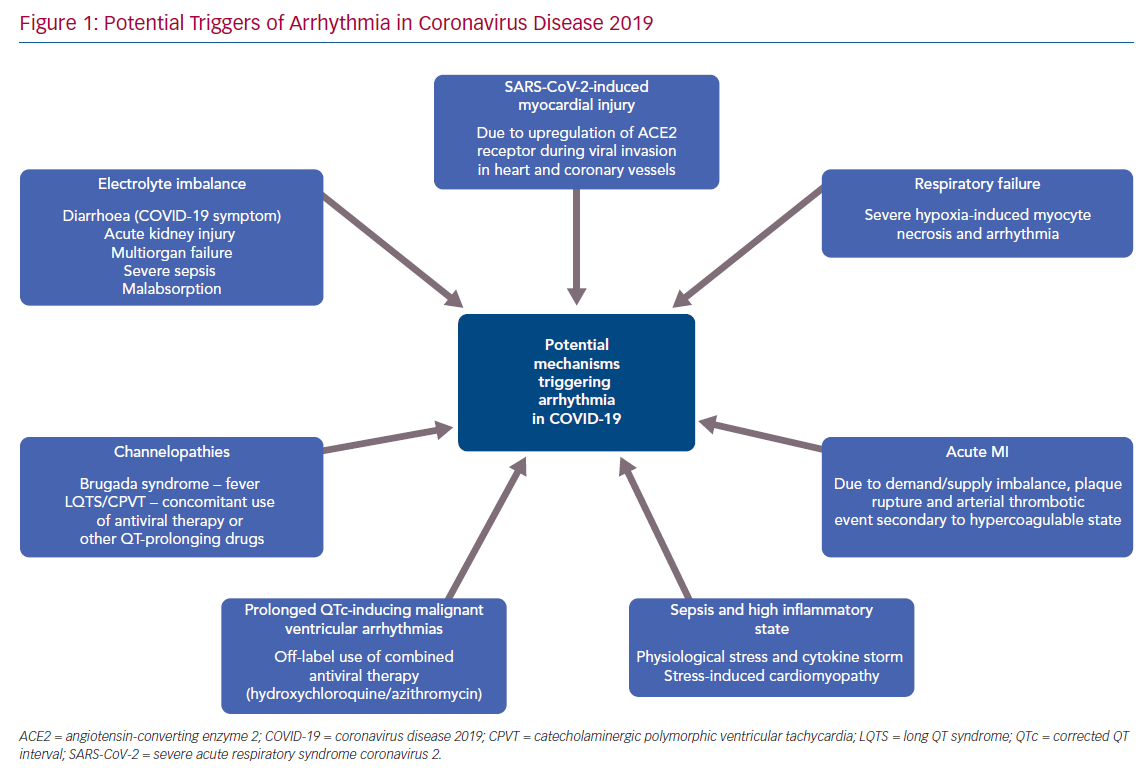
Arrhythmias
Arrhythmias are common in viral infections/sepsis and can occur during both the viral and inflammatory phases in COVID-19. There are several factors that may contribute to arrhythmias in the context of COVID-19 (Figure 1) including fever, sepsis, hypoxia, MI, myocarditis, stress-induced cardiomyopathy, electrolyte imbalance and multiorgan failure. A recent report from Wuhan noted arrhythmias in 16.7% of hospitalised patients with COVID-19, and in 44.4% of patients in the intensive care unit.14 However, the nature of the common arrhythmias has been poorly described in the literature so far.
Apart from achieving rate/rhythm control for AF/atrial flutter, anticoagulation for stroke prevention remains a challenging management strategy with regard to this disease entity. Uncontrolled activation of coagulation cascade following lung injury contributes to pulmonary inflammation in ARDS. As a result, extremely raised D-dimer levels are found in patients affected with COVID-19, with a substantial proportion affected with venous and arterial thromboembolism.13,15 There is no clear evidence as to whether all patients with the combination of severe COVID-19, new-onset AF and very high D-dimer would benefit from therapeutic anticoagulation irrespective of CHA2DS2VASc score due to the additional risk of thromboembolism associated with COVID-19. Being mindful of the bleeding risk in sepsis, particularly in the intensive care setting with the need for multiple central and arterial lines, careful consideration should be taken with an individualised management plan, balancing the possible therapeutic benefit against the risk of bleeding. Off-label antiviral therapy, such as lopinavir/ritonavir, has the potential for drug interaction with CYP3A-mediated direct oral anticoagulation drugs (DOAC; rivaroxaban and apixaban), thereby increasing the plasma DOAC levels. Hence either dose reduction or monitoring of plasma levels is essential to minimise the risk of bleeding.16–18
There is a small amount of emerging literature on the incidence of ventricular arrhythmias. In a single-centre study by Guo et al. of 187 hospitalised patients with COVID-19, the incidence was 5.9% for ventricular tachycardia (VT) and VF.19 VT/VF was also associated with elevated cardiac troponin levels and this raises the question of whether aggressive immunosuppressive therapy is warranted in patients with severe COVID-19. However, there are other known triggers that may induce ventricular arrhythmias in the context of COVID-19. Off-label antiviral therapies (hydroxychloroquine and/or azithromycin) that have been trialled in COVID-19 are well-known to cause prolonged QT interval and thus increase the risk of polymorphic VT.20 These drugs should be avoided outside the setting of a clinical trial, especially in those with underlying long QT syndrome (LQTS), and all healthy patients should be monitored with serial ECG on a periodic basis.
COVID-19 can also cause diarrhoea, malabsorption, acute kidney injury and electrolyte imbalance, which may pose a risk of ventricular and atrial arrhythmias.21–23 In addition, there is an increased risk of MI during the acute phase in patients with cardiac comorbidities, secondary to supply/demand imbalance, plaque rupture, severe hypoxia causing myocyte necrosis or arterial embolism due to hypercoagulable state, which can trigger malignant ventricular arrhythmias.24–26
Risk of Sudden Cardiac Death
There are no specific data on patients with channelopathies or inherited cardiomyopathies and COVID-19. However, COVID-19 could occur in patients with risk of sudden cardiac death with underlying diagnosed Brugada syndrome (BrS), congenital LQTS, catecholaminergic polymorphic ventricular tachycardia (CPVT), arrhythmogenic cardiomyopathy and hypertrophic cardiomyopathy. It seems likely that patients with cardiomyopathy may also have an increased risk of complications from COVID-19 and therefore should take precautions.
The primary concern with BrS is fever-induced malignant ventricular arrhythmia, and therefore fever should be aggressively treated with paracetamol. If this fails to control fever, patients should have continuous ECG monitoring and access to emergency cardiac support and defibrillation until the fever settles.
Although it is obvious that patients with a diagnosis of LQTS should not receive QT prolonging drugs, electrophysiologists will need to remind their colleagues that many patients with out-of-hospital cardiac arrest secondary to long QT, present as a result of iatrogenic intervention and, in the absence of drugs or electrolyte disturbance, may have a normal QT interval. A cautious approach is necessary when recruiting for COVID treatment trials. QTc interval should be monitored closely and QT-prolonging drugs should be stopped in the case of QTc >500 ms or if QTc increases by >60 ms from baseline.27 Electrolyte imbalances are often seen during acute illness, which should be promptly corrected particularly when associated with ECG changes. These include prolonged QTc (potential risk for torsades de pointes ventricular arrhythmia), visible U wave, atrial tachyarrhythmias and mild ST depression in severe hypokalaemia, QTc prolongation primarily by prolonging the ST segment in hypocalcaemia and QTc prolongation, atrial/ventricular ectopics and tachyarrhythmias in hypomagnesaemia.22
Patients with CPVT are often treated with beta-blockers and flecainide, which can interact with antiviral therapies, including hydroxychloroquine, azithromycin and lopinavir/ritonavir, causing serious arrhythmias. Intensivists will have a genuine challenge in managing these patients for whom beta-blockade is critical to avoid torsades de pointes, but for whom noradrenaline and other inotropes are necessary for maintaining blood pressure.
Phased Return of Elective Electrophysiology Procedures
Prioritising patient healthcare needs is of paramount importance during the pandemic in order to effectively utilise limited resources. Certain categories of EP procedures were deemed non-urgent to preserve resources to treat patients affected by COVID-19. Admissions with acute symptoms, such as complete heart block, symptomatic VT and symptomatic intractable arrhythmia, may not have significantly changed during the pandemic. However, the public may be reluctant to seek medical advice, and late presentations of acute cardiac events with their long-term sequelae have been described.28
In most healthcare systems, redeployment of staff has also had a huge impact on continuing EP elective work. Resuming elective work should be done in a safe and sustainable manner. Creating a model that suits the specific hospital is important to optimise the chances of a normal return to clinical services. We would recommend the following principles to guide recovering services.
Minimising Patient Exposure to the Public and Healthcare Workers
It is obvious that patients travelling to receive healthcare advice or treatment are emerging from isolation and are particularly exposed if interacting with healthcare services that also manage COVID patients. Patient exposure can be minimised by establishing ‘clean’ services working on the principle that if the patient does have to meet a healthcare professional, then that professional should be dedicated to only looking after patients who do not have COVID symptoms. This can be achieved by:
- All clinic consultations and preadmission visits being carried out via telephone or online video consultation.
- All prolonged ECG monitoring (Holter) being posted to the patient and instructing them by phone how to attach them.
- Increased use of patient administered and owned ECG monitoring. Apple Watch and AliveCor Kardia are the most well-known, but there is a plethora of equally effective and cheap technologies available for online purchasing.
- Posting pre-admission blood tests and swabs to the patient. Some blood tests can be delivered via point-of-care or finger prick systems, avoiding the patient having to attend a clinical service. COVID-19 swabs are notoriously difficult to perform adequately by the general public and may be harder to deliver reliably.
- All device follow-ups being performed remotely when possible. At St Bartholomew’s Hospital, we have been sending out remote monitoring systems to patients with remote-capable devices, but some patients with legacy devices will have their follow-up deferred if they are at low risk and asymptomatic.
- In-person investigations (echo and MRI) being delivered in isolated or community services, remote from acute COVID-19 care services.
Appropriate Triage of Elective Care
Patients with time-critical conditions should be given priority, regardless of their risk of exposure to the general public. This includes those with complete heart block and pacing-dependent patients reaching the end of their device battery life.
Persistent AF is our biggest dilemma because although most of these patients have no prognostic benefit from ablation, they are time critical in that delaying their ablation procedure results in poorer outcomes. We have been assessing their risk of COVID-19 and their AF symptoms and making joint decisions with the patients about when and if they have their procedure.
Other patients with non-time critical conditions (symptomatic supraventricular tachycardia) are now being given dates for procedures, particularly if they are at low risk of COVID-19 and/or they are having to seek emergency room assistance for their condition.
This process takes a lot of time and discussion with patients. Therefore, although our catheterisation labs are not as busy, our clinicians are, because they are spending time making these difficult decisions and prioritising. This also has created a lot of work for administrators, who are responsible for patient scheduling.
Minimising Procedure Risks to Staff
While it is good practice to screen patients for COVID-19 ahead of admission, we can never be certain that patients will be free of the virus. There are specific considerations that can be given to EP procedures to help reduce risk to staff, primarily around minimising aerosolising procedures. This could include:
- minimising procedures under general anaesthetic;
- minimising the use of transoesophageal echo; and
- minimising use of diathermy.
There is some evidence that diathermy can produce particles containing viral material. Vaporisation of protein and fat from heated tissues causes surgical smoke, which contains chemicals, biological particles, viruses and bacteria. This smoke from tissue pyrolysis is known to be potentially hazardous, especially at the current time.29,30 For many decades we implanted devices without the use of diathermy and we would suggest that this can be avoided or minimised for the moment.
Other Considerations
The use of hospitals not normally involved in national healthcare provision, e.g. independent or field hospitals, may provide capacity for investigations or procedures, not normally available.
Staff should have access to appropriate personal protective equipment and training in proper hygiene, donning and doffing it, but should use this only in higher risk situations in order to to preserve supplies.
Conclusion
COVID-19 can cause a wide range of cardiovascular complications, of which arrhythmias are one of the most common. Furthermore, the pandemic has had an unprecedented impact on healthcare systems globally, certainly affecting elective procedures. However, on the positive side, digital health and telemedicine have played an important role in EP during the pandemic, and will change the way we work, well beyond the end of the COVID-19 crisis.