Heart failure (HF) and AF are two conditions that are increasing in prevalence worldwide.1,2 They frequently co-exist and in recent years, the clinical and physiological intersection between arrhythmia and HF has become an area of renewed interest, particularly as interventional treatments for rhythm disorders have advanced and moved into the mainstream of cardiac management. In particular, AF, the most frequently encountered cardiac arrhythmia, is now no longer considered as a passive bystander in the setting of HF, but rather an active determinant of clinical outcome,and in some circumstances, the critical driver of the HF itself. 3–5 In this modern context, it is important to re-evaluate the role of existing medical and interventional strategies in the management of patients with co-morbid AF and HF.
Older Studies and Their Limitations
The management of AF in HF has been coloured by two early large randomised trials which demonstrated no mortality benefit of pharmacological rhythm control over rate control. In what is still the largest randomised study ever conducted in AF, the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial evaluated overall mortality in 4,060 patients with varying AF burdens, 26% of whom had HF.6 Patients were randomised to a strategy of pharmacological rhythm control (n=2,033) or rate control (n=2,027). No difference was seen between the groups at 5 years, and there was a trend towards a worsened outcome in the rhythm control group (p=0.08).
Less well-known is a detailed sub-analysis of the data showing that the presence of sinus rhythm (SR) was associated with a significantly reduced mortality (HR 0.54; p<0.0001) that was largely offset by the increased mortality associated with anti-arrhythmic medical therapy to achieve SR, predominately (63%) amiodarone (HR 1.41; p=0.0005).7 Additionally, HF symptoms were also significantly improved with rhythm control.8
Roy et al. randomised 1,376 patients with HF (left ventricular ejection fraction [LVEF] 27 ± 6%) to rhythm (n=682) or rate control (n=684), and also showed no difference in mortality (p=0.59).9 There are two crucial limitations of this study, which largely reflect the limitations of rhythm control management at the time. Firstly, amiodarone, known to be associated with increased mortality, was the rhythm control agent used in the majority (84%) of patients. Ablation was used in only 3.2% of patients. Secondly, it is important to note the study compared treatment strategies (rhythm control to rate control) and so was inherently limited by the poor efficacy of medical rhythm control strategies to maintain durable SR. At 5 years follow-up, only 42% of patients in the rhythm control arm were free from AF. This limited the study’s ability to assess the effect of durable SR upon outcome. Despite these limitations, these studies continue to influence the current clinical guidelines for management of AF, including in those with concurrent HF.
Catheter Ablation for AF in Heart Failure
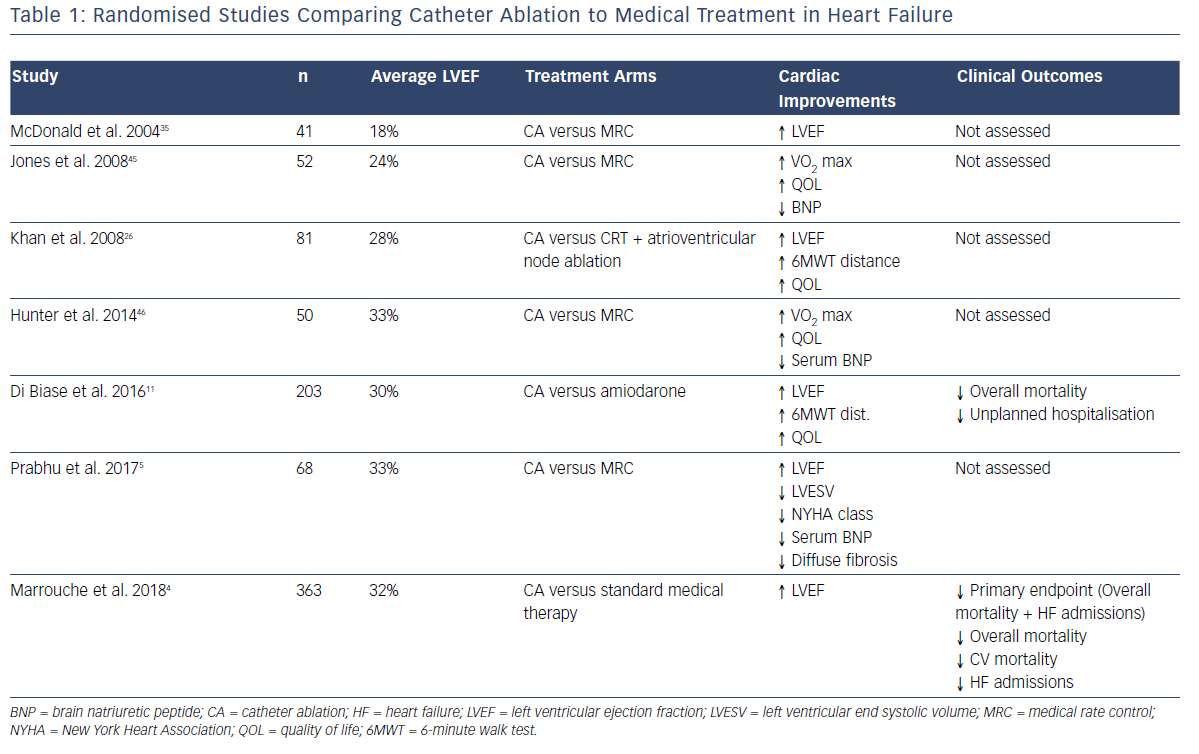
The recent advent of catheter ablation as a mainstream treatment for AF has allowed the restoration of SR with improved efficacy and without the toxicities of long-term anti-arrhythmic therapy. Consequently, a consistent body of evidence has been developed demonstrating the benefits of catheter ablation in patients with systolic HF compared to standard medical therapy. This has recently been expertly reviewed by Mukherjee et al.10 Table 1 summarises the existing randomised data comparing catheter ablation to medical therapy (either rhythm or rate control). Consistent improvements in ejection fraction, functional capacity (both objective and subjectively assessed), biomarkers and objective quality of life measures have been demonstrated.
The Ablation vs. Amiodarone for Treatment of Atrial Fibrillation in Patients with Congestive Heart Failure and an Implanted ICD/CRTD (AATAC-AF) study specifically compared the efficacy of a strategy of rhythm control with catheter ablation to rhythm control with amiodarone in patients with HF, by randomising 203 patients to either strategy.11 The ablation arm demonstrated unequivocal superiority in terms of maintaining SR (70% versus 34% at 24 months; p<0.001), in addition to reduced mortality (8% versus 18%; p=0.037) and unplanned hospitalisations (RR 0.55; 95% CI [0.39– 0.76]).
The Catheter Ablation versus Standard Conventional Therapy in Patients with Left Ventricular Dysfunction and Atrial Fibrillation (CASTLE-AF) study compared catheter ablation to standard medical therapy in patients with HF and was specifically powered to evaluate the hard-clinical endpoints of mortality and HF hospitalisation.4 In addition to significantly fewer patients randomised to catheter ablation meeting the primary endpoint (28.5% versus 44.6%; HR 0.62; p=0.007), individual secondary endpoints including overall mortality (HR 0.53; p=0.01) cardiovascular death (HR 0.49; p=0.009) and unplanned HF admissions (HR 0.56; p=0.004), all reached significance in favour of catheter ablation.4 Several recent meta-analyses have consistently shown improvements in ejection fraction, quality of life, functional capacity, hospitalisation and mortality.12–14
Recently, the much-anticipated results of the Catheter Ablation Versus Anti-arrhythmic Drug Therapy for AF (CABANA) trial were presented at the 2018 HRS Late Breaking Clinical Trials Session. That trial randomised 2,204 patients with AF to either catheter ablation (n=1,108) or standard medical therapy (n=1,092). Although the primary endpoint (composite of all-cause mortality, disabling stroke, serious bleeding or cardiac arrest) was negative for the overall study (p=0.30), those patients undergoing catheter ablation with symptomatic HF (NYHA II+), had a significant 49% reduction in the primary endpoint.
The Interaction of AF and Heart Failure
AF and HF share several pathophysiological mechanisms, each of which promote the progression of the other. AF drives HF by three primary mechanisms:
- tachycardia;15
- ventricular irregularity;16 and
- the loss of atrial contractile function.17
Irrespective of its aetiology, HF creates a physiological environment which facilitates the development and progression of AF through adverse atrial remodelling.18–19 This occurs through:
- raised filling pressures;20
- abnormal calcium handling;21 and
- the activation of neural-hormonal pathways which promote atrial stretch and fibrosis.22
For this reason, AF and HF frequently co-exist with reported rates as high as 35% in some studies.23 Disentangling the “chicken and egg” relationship between the two can be challenging for the treating physician, particularly as the symptoms of both conditions are often non-specific (such as exertional dyspnoea and fatigue) with palpitations often absent. In patients with dilated cardiomyopathy, the presence of AF at the time of initial presentation with HF has been reported as high as 68%.5 Nonetheless, attempting to ascertain the contributory significance of the AF to the HF is crucial as the elimination of AF in some patients, may have a dramatic impact upon cardiac function.
AF-mediated Heart Failure
The ability of AF to cause systolic dysfunction has been somewhat underappreciated,particularly where the cause of HF is uncertain (often classed as idiopathic).24 The recently reported Catheter Ablation Versus Medical Rate Control in AF and Systolic Dysfunction (CAMERA-MRI) study evaluated 66 patients with persistent AF and LVEF ≤45%, who were randomised to either catheter ablation or continuing ongoing medical rate control (MRC).5 All patients were on established anti-failure medical therapy and had optimal MRC at baseline. Patients underwent cardiac MRI at baseline and 6 months post randomisation. At 6 months, the catheter ablation group had substantially improved LVEF compared to the MRC arm (18.3 % improvement versus 4.4%; p<0.0001). Furthermore, 71% patients undergoing catheter ablation with no evidence of scarring (or late gadolinium enhancement) on baseline MRI imaging, had normalised LV function by 6 months, suggesting this imaging feature may identify those patients with a true underlying AF mediated cardiomyopathy.
Limitations of Medical Rate Control
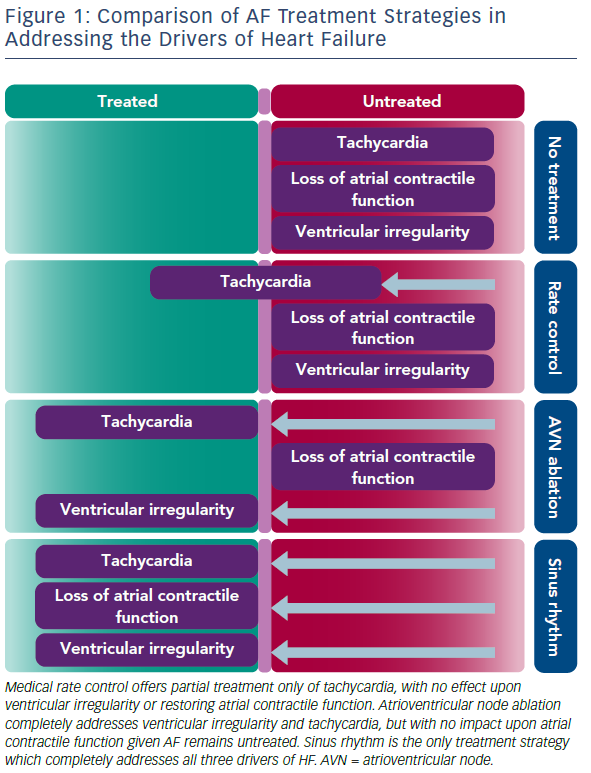
Importantly, the benefits of restoring SR for improving ventricular function seen in the CAMERA-MRI study were demonstrated even in well-managed rate-controlled AF. Average ventricular rates were within guideline criteria before randomisation and further improved in the MRC arm during the study period. While the concept of tachycardia-mediated cardiomyopathy has been well-described, the ability for irregular ventricular activity and/or the loss of atrial contractile function to mediate reduced systolic function in the absence of rapid rates is relatively novel. Hsu et al. first described significant improvements in LVEF post catheter ablation even in patients adequately rate-controlled at baseline.25 Furthermore, in a small randomised study comparing the restoration of SR with catheter ablation to the ultimate rate control of pacing and AV node ablation, Khan et al., demonstrated a greater improvement in LVEF in those in SR.26 Figure 1 illustrates a likely hypothesis for this.
SR is the only treatment strategy that completely treats all three drivers of HF, thus explaining its benefit over pacing and atrioventricular node (AVN) ablation which are still unable to restore atrial contractile function.26 As demonstrated in the CAMERA-MRI study, even maximal MRC is unable to match the average ventricular rates achieved by the restoration of SR. As such, MRC effectively only partially treats the tachycardia component, with no impact on the other mechanisms. At 6 months, mean heart rate was significantly lower in the catheter ablation group (all of whom were in SR, compared to the MRC group, all of whom were in AF (67 ± 9.1 versus 86 ± 14 BPM; p<0.0001). Similar findings were seen for resting and post exercise heart rates.5 Additionally, in a sub-study of CAMERA-MRI, the restoration of SR also resulted in a regression of adverse ventricular remodelling (ventricular diffuse fibrosis) compared to MRC, suggesting an additional benefit of SR in the context of HF.27
Other Types of Heart Failure
What of those patients with known underlying causes of HF, such as ischaemic cardiomyopathy? Ostensibly in such patients, the degree of ventricular impairment would be determined largely by the underlying structural heart disease, e.g. the extent of myocardial infarct, rather than the impact of AF, although the associated presence of AF may worsen the ventricular function in these patients. The current literature offers minimal guidance in this area. Hsu et al. published the outcomes for 58 patients undergoing catheter ablation, compared to 58 patients without HF. They found no impact of underlying structural heart disease upon outcome.25 Similarly, the CASTLE-AF study included 46% of patients with ischaemic cardiomyopathy and found no difference in the primary outcome, even when stratified by HF type (p=0.56).4 In the CAMERA-MRI study, those patients with non-ischaemic cardiomyopathy with evidence of scarring using late gadolinium enhancement on cardiac MRI, still had a significant improvement in LVEF following catheter ablation, although the magnitude of such improvement was proportional to the extent of scarring present at baseline.5 In contrast, recent multicentre series and a meta-analyses of catheter ablation in HF, suggested that the presence of structural heart disease and fibrosis predicted a worse long-term outcome with respect to LVEF improvement, freedom from AF and mortality.12,28,29
Until more prospective studies are completed, which specifically compare clinical outcomes of patients with known structural heart disease including extensive fibrosis, the extent to which this feature should influence treatment decisions is unclear. Nonetheless, given the results of the CAMERA-MRI study, the presence of minimal fibrosis should likely not deter from an ablation strategy.
What Constitutes Success in Catheter Ablation for HF?
The vast majority of AF encountered in the setting of HF is persistent, particularly in the circumstance where AF is the primary driver of the left ventricular dysfunction. Yet persistent AF outcomes post catheter ablation are consistently reported as inferior to those of paroxysmal AF. Such pessimism about outcome may deter physicians from tackling these challenging cases. There are two important factors to consider here. Firstly, although the gold standard definition of AF recurrence is defined as any AF or AT >30 seconds for clinical trial outcomes,30 this definition of success likely has little utility in the setting of patients with predominately long-standing persistent AF.
The Substrate and Trigger Ablation for Reduction of AF Trial (STAR-AF II), is the largest clinical trial of patients with persistent AF.31 It followed up 589 patients for 18 months with weekly transtelephonic rhythm monitoring, in addition to regular Holter monitoring. Procedural success improved from 44%–75%, simply by altering the cut-off for defining recurrence from >30 seconds to >24 hours of AF.32 Importantly, even 24 hours of AF over 18 months of follow-up still equates to an AF burden of 0.002%.
More recently, the utility of the traditional cut-off of >30 seconds has been questioned by Steinberg et al. who evaluated the 12-month outcomes for 615 patients with dual chamber cardiac implantable electronic devices with at least one episode of AF >30 seconds detected at baseline.33 They found that AF between 30 seconds and 2 minutes was a poor predictor of clinically meaningful AF with 36% of patients experiencing no further episodes of AF >2 minutes over the study period.
Importantly, recent trials of catheter ablation in HF have measured AF burden in addition to the conventional definition of recurrence.4–5,11 A post hoc analysis of the CASTLE-AF study, in which all participants had a dual chamber ICD or cardiac resynchronisation therapy (CRT) device implanted, demonstrated that recurrence (determined by AF >30 seconds) had no statistical relationship with the primary endpoint.34 In contrast, an AF burden of 6% or less, predicted a 2.5–3.3-fold freedom from the primary endpoint, compared to those with AF burden >6%. In that study, although the average AF burden in the catheter ablation arm at final follow-up was 27%, the median AF burden was 0%, suggesting the majority of patients in the catheter ablation arm had actually no clinically significant AF, and the reported average may have been driven by a smaller number of patients with very high AF burdens.
Thus, AF burden reduction, rather than freedom from recurrence, is probably a far more useful treatment aim, and reported high rates of recurrence should not deter from the use of catheter ablation as an anti-heart failure treatment in patients with persistent AF and HF. However, the exact magnitude of burden reduction required to derive clinical benefit is likely yet to be fully elucidated.
Limitations of Clinical Trials
It is worth noting that despite consistency of findings in recent clinical trials, there are important limitations that should be noted. With the exception of CASTLE-AF and AATAC-AF, most studies have had modest patient numbers. The findings are really only applicable to candidates with stable, well-compensated HF who were otherwise suitable for catheter ablation. This may have resulted in selection bias towards less severe HF phenotypes. The randomised study of catheter ablation and MRC by Macdonald et al., which included generally sicker patients than other studies (average LVEF 16%; 91% NYHA III, average of 19 previous hospitalisations and longer average AF durations of 4–5 years), showed poor success rates (50% restoration to SR) and did not show a significant improvement in LVEF on cardiac MRI (CMR).35
Additionally, a secondary analysis of patients in the CASTLE-AF study highlighted that those with LVEF <25% had a significantly higher occurrence of the primary endpoint (mortality or unplanned HF-related admission) compared to those with LVEF ≥25%.4 These findings suggest that patients with more severe HF may not benefit from catheter ablation. Finally, given the nature of the intervention, blinding of study participants to treatment allocation was not possible. However, in many studies, the endpoint adjudicators were blinded to treatment allocation.
Ongoing Trials of Catheter Ablation in Heart Failure
There are three recent large randomised controlled trials evaluating catheter ablation in patients with HF. The AF Management in Congestive Heart Failure With Ablation (AMICA; NCT00652522) study was completed in 2017 and compared LVEF at 12 months following ablation or MRC or atrioventricular (AV) node ablation in patients with persistent AF, LVEF <35% and NYHA class II/III.
The Rhythm Control – Catheter Ablation With or Without Anti-arrhythmic Drug Control of Maintaining Sinus Rhythm Versus Rate Control With Medical Therapy and/or Atrio-ventricular Junction Ablation and Pacemaker Treatment for AF (RAFT-AF; NCT01420393) study is evaluating mortality or unplanned HF-related hospitalisation in patients with paroxysmal or persistent AF, LVEF <45% and NYHA II/III heart failure randomised to catheter ablation or rate control (pharmacological or AVN ablation).
The Ablation of AF in Heart Failure Patients (CONTRA-AF; NCT03062241), study is evaluating mortality or unplanned HF-related hospitalisation in patients with paroxysmal or persistent AF, LVEF <35% and dual chamber ICDs, or CRT-D in situ in patients randomised to balloon cryoablation for AF or medical therapy.
The publication of these studies in due course will greatly improve our understanding of the role of catheter ablation in HF.
Risks, Complications and Cost-effectiveness of Catheter Ablation
Despite the presence of systolic dysfunction, several prospective and retrospective analyses have shown generally low complication rates in patients with concurrent AF and HF,4–5 or at least rates comparable to patients without HF.36 Although not overtly apparent in large published data sets, perceivably patients with more severe HF phenotypes may have higher rates of thrombo-embolic complications.35,37
Particular attention should be paid to pre-procedural, intra-procedural and post-procedural anti-coagulation with uninterrupted anti-coagulation strategies with either vitamin K antagonists or direct-acting oral anti-coagulants (DOACs) being the preferred option, to further minimise the risk of thrombo-embolism.38–40 As with all AF ablation procedures, detailed discussion of the recognised risks of AF ablation (including stroke, cardiac tamponade, atrio-oesophogeal fistula, groin complications and adjacent nerve injury), should be central to informed consent.
Cost-effectiveness analyses of AF ablation are generally lacking. However, the weight of data suggests that the cost:benefit ratio favours ablation in younger, highly symptomatic patients with poor response to anti-arrhythmic medications, and frequent hospitalisations.40 This most ardently applies to patients with concurrent HF who frequently fail medical therapy and frequently require hospitalisation in the setting of AF-mediated acute on chronic exacerbations of HF. Nonetheless, a specific cost-effectiveness of analysis of ablation in AF and HF patients is yet to be formally undertaken.
Limitations of Current Clinical Guidelines
Current guidelines are yet to be updated to reflect the emerging role of catheter ablation in the setting of HF. The European Society of Cardiology and the American College of Cardiology/American Heart Association guidelines have no specific recommendations for the role of catheter ablation in HF.41,42 In contrast, the National Institute for Health and Care Excellence guideline on the management of AF suggests rhythm control should be the firstline treatment for patients in whom HF is “thought to be primarily caused by the AF”,43 leaving open an initial ablation strategy management option. Recent trial data have heralded a call for guidelines to be updated in the near future.24,44
A New Treatment Algorithm for Catheter Ablation in Heart Failure
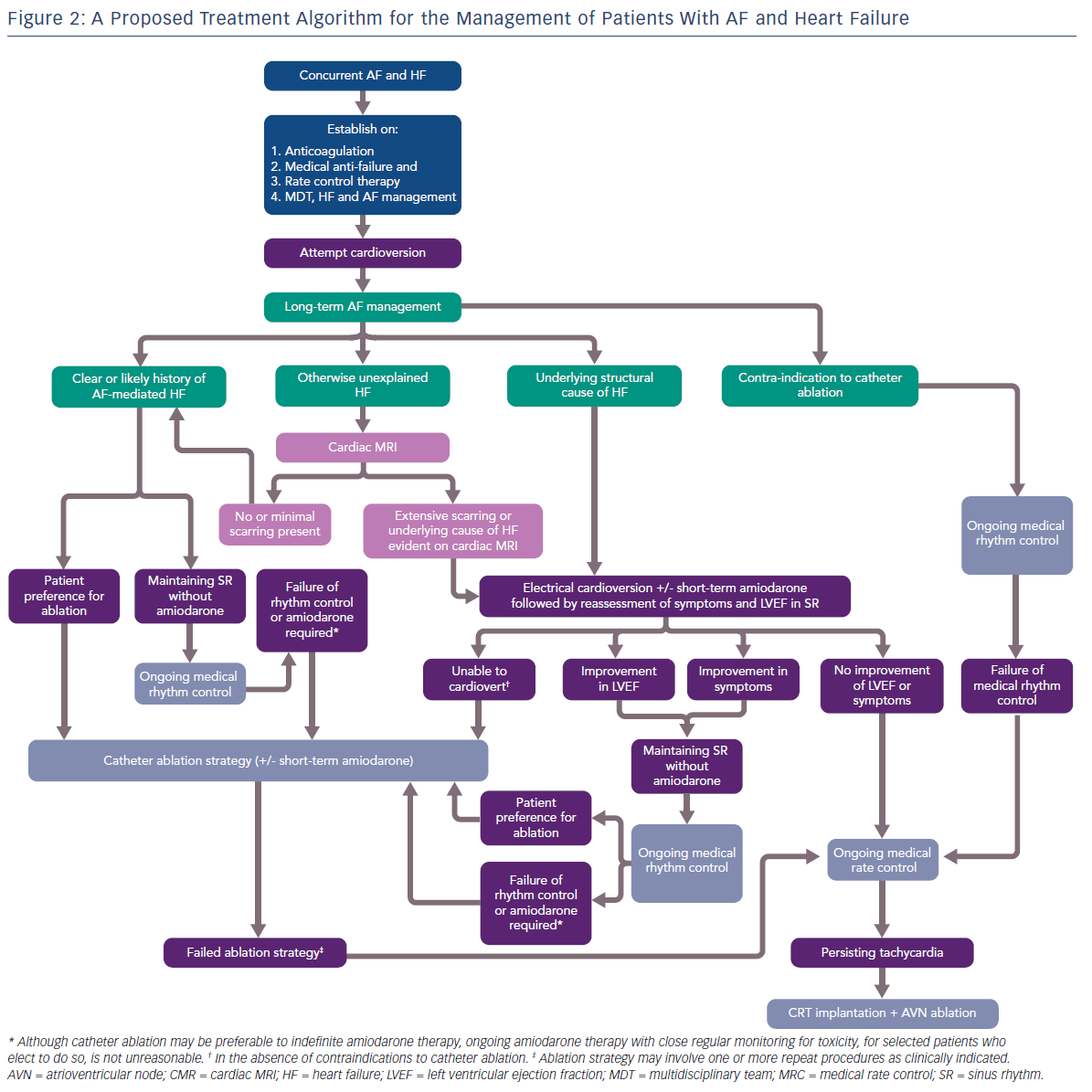
Given that the results of contemporary clinical trials are yet to be reflected in practice management guidelines, we attempt to provide some pragmatic guidance to manage patients presenting with co-morbid AF and HF, with a focus on the role of catheter ablation (Figure 2).
Priorities for patients presenting with co-morbid AF and HF are the commencement of anti-coagulation, medical anti-failure pharmacological therapy, suppression of overt tachycardia and the establishment of a multidiscliplinary HF team, ideally including a HF cardiology specialist and HF nurse. Acute management of AF with electrical cardioversion is preferable at this stage. With regards to long-term management of AF, patients can divided into two main groups.
Firstly, patients with a clear clinical history of AF-mediated cardiomyopathy may present with co-diagnosis of AF and HF, may have documented normal LV function while in SR, and not have underlying structural heart disease. Patients not wanting to take anti-arrhythmic drug (AAD) therapy, those who fail first-line AAD therapy or who can only maintain SR with amiodarone, should be offered catheter ablation.
Secondly, in patients with a known cause of HF, the contribution of AF to the LV dysfunction and/or symptoms should be clarified with the restoration of SR with the assistance of short-term amiodarone. Those patients demonstrating a significant improvement in LVEF and/or symptoms should be considered for catheter ablation as an alternative to long-term amiodarone therapy.
In those patients where the cause of HF is unclear, cardiac MRI is a useful tool for further stratification. Based on the findings of the CAMERA-MRI study, patients with no or minimal scarring should be considered to have an underlying AF-mediated cardiomyopathy and managed as per patients in the first group. Those patients with extensive scarring, or where CMR identifies an underlying cause of HF, e.g. cardiac sarcoid, should be managed as those in the second group.
Patients deriving no benefit in symptoms or LVEF improvement from SR, or who eventually fail a strategy of catheter ablation, should have ongoing MRC, with the option of CRT implantation and AVN ablation available to those with persistent tachycardia. Catheter ablation should be performed by experienced operators in high volume centres with specialised expertise in complex ablation and the management of advanced cardiomyopathy. It also should be noted that there are no current data regarding the safety and efficacy of cryoablation as an ablation strategy in patients with HF, and the vast majority of clinical trials in AF and HF have utilised RF-based catheter ablation. Similarly, as with persistent AF, the optimal ablation strategy beyond PVI is unknown.
Conclusion
There is now a considerable body of evidence suggesting that the maintenance of SR while avoiding long-term AADs such as amiodarone, in patients with AF and HF leads to improved clinical outcomes with respect to LV function, symptoms, hospitalisation and mortality. Catheter ablation provides this and should be considered as an important part of HF management in these patients. The traditional measures of success following catheter ablation, namely AF recurrence, likely have little relevance to long-term clinical outcome in these patients and catheter ablation should not be withheld as a treatment option for this reason alone. Instead, catheter ablation should be viewed as a tool to control AF burden and consequently improve HF and clinical outcomes. Additionally, cardiac MRI may be utilised as an important stratification tool in identifying patients with a likely AF-mediated cardiomyopathy and therefore likely to derive the most benefit from rhythm control with catheter ablation.
Clinical Perspective
- An increasing body of evidence suggests that catheter ablation for AF is effective, feasible and safe with improvements in symptoms, ventricular function, reduced heart failure-related hospitalisation and mortality.
- Although yet to be formally reflected in clinical guidelines, catheter ablation for AF in patients with AF should be strongly considered in patients with heart failure, particularly those unable or unwilling to take anti-arrhythmic drug therapy, such as amiodarone.
- In patients with AF and an otherwise unexplained cardiomyopathy, cardiac MRI can be a useful stratification tool. The absence of scarring is suggestive of an underlying AF-mediated cardiomyopathy, even in the setting of adequate rate control, and these patients will gain the most benefit from catheter ablation.