The most common sustained cardiac arrhythmia is AF, which is associated with increased risk of stroke and heart failure, and represents a significant global health burden.1 Pulmonary vein isolation (PVI) is the standard treatment for symptomatic, drug resistant paroxysmal and persistent AF.2 Radiofrequency (RF) ablation, the most widely used ablation technique, aims to achieve circumferential ipsilateral PVI by creating contiguous and transmural point-by-point lesions.3 However, pulmonary vein (PV) reconnection following ablation leads to AF recurrences, necessitating repeat ablations, putting patients at increased risk of complications and decreasing the cost-effectiveness of the procedure. Rates of ablation procedures have risen over the last decade,4 but in order for catheter ablation to become standard first-line treatment for AF patients, there is a need for higher and reproducible single-procedure success rates.
CARTO VISITAG™ Module with Ablation Index (Biosense Webster) is a new technology providing visual lesion representation based on the integration of power, contact force and time parameters to be displayed on the CARTO® 3 System (Biosense Webster). This index was developed to simplify and standardise the workflow for ablating patients with paroxysmal AF (PAF) and support electrophysiologists using the CARTO SMARTTOUCH™ Technology (Biosense Webster) in reproducing their own successful ablation strategy to achieve PVI. This article will review the literature on optimising efficacy and outcomes with the use of contact force (CF), describe the ‘CLOSE’ protocol – an ablation strategy guided by the CARTO VISITAG Module with Ablation Index – and discuss the need for a reproducible and standard RF ablation strategy.
Contact Force: A Critical Determinant of Lesion Size
RF current at the electrode tissue interface leads to tissue injury. Although RF current cannot be measured directly, RF power and duration can be measured and altered. Because equivalent power and duration applications do not give equivalent lesion sizes, lesion size cannot be reliably assessed in real time. CF is a key parameter to controlling lesion size. It has been clearly demonstrated that CF is a determinant of lesion size; at constant power, tissue temperature and lesion size increased with CF. In addition, the incidence of steam pop and thrombus increased with CF.5
Shah et al.6 have examined the delivery of RF energy during constant contact at 20 g, variable contact with a 20 g peak and 10 g nadir and intermittent contact with a 20 g peak and 0 g nadir with loss of contact. The area under the CF curve was calculated as the force-time integral (FTI). The measured FTI was highest during constant contact, intermediate during variable contact and lowest during intermittent contact and correlated linearly with lesion volume. An estimation of lesion size was possible by combining CF and the duration of RF delivery.6
Ikeda et al. assessed the relationship between lesion size and CF by measuring electrogram amplitude, impedance and electrode temperature in a beating canine heart.7 At constant power and duration of RF delivery, lesion size increased significantly with increasing CF (p<0.01). There was a poor correlation between peak electrode temperature and lesion size. The decrease in impedance during the RF application correlated well with lesion size for lesions in the left ventricle but less well for lesions in the right ventricle.7
Therefore, CF is a critical determinant of lesion size. Increasing CF at constant power increases lesion size. Steam pops are uncommon at CFs <20 g and RF power/duration levels used in the clinical setting. The appropriate CF needed to achieve transmurality with 25–30 W delivery in the thin-walled atrium is lower than for the ventricle.
Clinical Impact of Contact Force Technologies
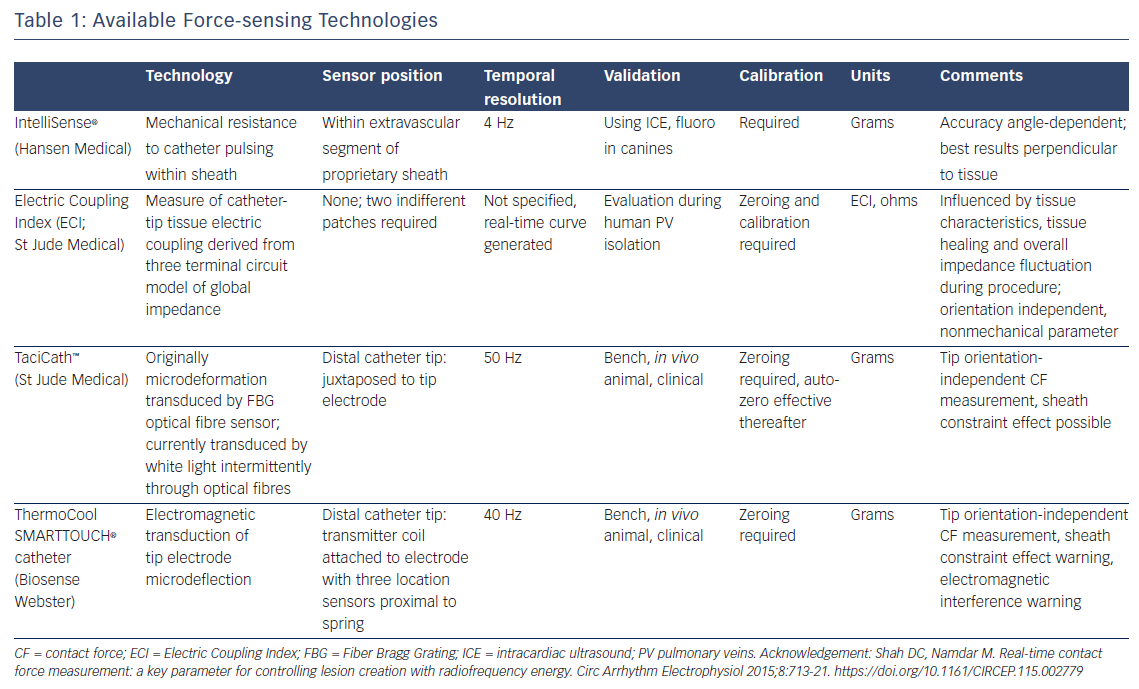
Currently tested modalities include the IntelliSense® force sensing technology8 (Hansen Medical), Electric Coupling Index (ECI; St Jude Medical), TactiCath™ (St Jude Medical) and ThermoCool SMARTTOUCH® (Biosense Webster) catheters (see Table 1). Assessment of ECI remains challenging. It requires scaling to the individual patient and is repeated every 30 minutes; therefore, it is difficult to compare between patients. In addition, ECI and the nature of its changes during tissue contact are not completely understood. It lacks precise distinction between levels of contact, and may be challenging to interpret at sites of previous ablation injury or spontaneous atrial fibrosis. Both the TactiCath and ThermoCool SMARTTOUCH have been demonstrated to be highly accurate in assessing tip-tissue contact, albeit using quite different force assessment technologies.9
A systematic review and meta-analysis of nine non-randomised studies involving 1,148 patients aimed to evaluate the clinical impact of CF technology. The primary endpoint was relative risk of AF recurrence at follow up. RF duration, total procedure length and fluoroscopy exposure were assessed as secondary outcomes. Compared with standard technology, the use of CF technology showed a 37 % reduction (relative risk [RR] 0.63; p=0.01) in AF recurrence at a median follow up of 12 months and a 7.3-minute reduction (p=0.03) in RF use during ablation. However, there was no significant difference in total procedure length and fluoroscopy exposure between the two groups.10 This analysis also included small early studies.
A multicentre study of 600 patients evaluated the impact of CF-sensing technology on medium-term outcome of AF ablation. First-time AF ablation procedures employing CF-sensing catheters from four centres were matched retrospectively to those without CF-sensing catheters in a 1:2 manner by type of AF. The primary outcome measure was freedom from recurrence of atrial arrhythmia, with fluoroscopy time as a secondary outcome measure. A total of 600 AF ablation procedures (200 using CF and 400 using non-CF catheters) were performed. Of these, 46 % were PAF, 36 % persistent AF and 18 % long-lasting persistent AF. The follow-up duration was 10.0–11.4 months. Similar complication rates were reported in both groups. The use of a CF-sensing catheter independently predicted clinical success in ablating PAF, but not non-PAF. In all cases, the use of CF catheters was associated with reduced fluoroscopy time.11
Further insights can be derived from non-comparative studies. The first published study of the use of CF-ablation catheters in humans was the Touch+™ for Catheter Ablation (TOCCATA) study. Patients with PAF underwent PVI using the TactiCath catheter. All patients (n=5) treated with an average CF of <10 g experienced recurrences, whereas 80 % of the patients treated with an average CF of >20 g (n=10) were free from AF recurrence at 12 months. The investigators concluded that CF achieved correlates with clinical outcome.12
In the prospective, multicentre, Prospective Safety Assessment of the ThermoCool SMARTTOUCH SF Family of Contact Force Sensing Catheters for the Radiofrequency Ablation Treatment of Drug Refractory Symptomatic Paroxysmal Atrial Fibrillation (SMART-AF) study, 160 of 172 enrolled patients from 21 centres underwent AF ablation using the ThermoCool SMARTTOUCH catheter. The majority of procedures employed between 10–25 g. Investigators remained within their individually defined prespecified ranges 73.3 ± 18.4 % of the time. Success rate after 1 year follow up was 74 % when the operator remained in the preset CF range. This improved to 81 % when the investigator stayed within their preselected CF range ≥80 % of the time. Further subanalysis showed that when the CF was within investigator selected range ≥85 % of the time, success rate increased to 88 %.13 Stable CF during RF ablation increases the likelihood of 12-month success.
What About Randomised Trials?
The importance of optimal contact has been demonstrated in the prospective randomised non-inferiority Ablation Catheter Study for Atrial Fibrillation (TOCCASTAR) study, which compared the TactiCath catheter with a non-CF-sensing catheter (NAVISTAR® ThermoCool®; Biosense Webster) in 300 patients undergoing catheter ablation of PAF.14 When the CF arm was stratified into optimal CF (≥90 % ablations with ≥10 g) and non-optimal CF groups, effectiveness was achieved in 75.9 % versus 58.1 %, respectively (p=0.018).
In a recent randomised trial performed at seven UK centres, 117 patients undergoing first-time PAF ablation were randomised to ablation with (CF-on) or without (CF-off) CF data available to the operator. A reduction in acute PV reconnection rates was seen in the CF-on group (22 % versus 32 %, p=0.03) but there was no significant difference in 1-year success rates (49 % versus 52 %, p=0.9). Furthermore, there was no difference in major complication rates, procedural times and fluoroscopy times. The investigators concluded that CF data availability as used in this study was associated with improved catheter control but did not impact on the clinical outcome. This confirms that CF is only one of multiple factors that determine lesion efficacy and safety.15
Three other small prospective randomised studies have compared CF versus non-CF-sensing catheter ablation. These studies have shown that some operators are able to estimate CF with a high degree of accuracy when CF information is not available and may explain why, when the CF information was available, none of these studies demonstrated a statistically significant improvement in long-term outcomes.16–18
Despite the lack of superiority demonstrated by randomised studies, some experts consider their personal experience with CF catheters to be so favourable they would “consider it almost unethical to perform an AF RF ablation with a non-CF-sensing catheter”.19 It is possible that the failure to demonstrate unequivocal benefit can be explained by study design, variability in the catheter and mapping systems used and the absence of target guidelines at the procedural outset. In addition, the operators in the trial were highly skilled.19 Furthermore, although catheter-tissue contact is the single most important determinant of RF lesion formation to which there is access in the electrophysiology laboratory, consistency and contiguity of lesions is more important than absolute level of contact per lesion above a threshold. Objective lesion representation tools such as CARTO VISITAG Module with Ablation Index could provide the means to translate biophysical parameters including CF into a reproducible and reliable delivery of RF lesion sets.
Towards A Standardised Protocol For Durable PVI: The CLOSE Protocol
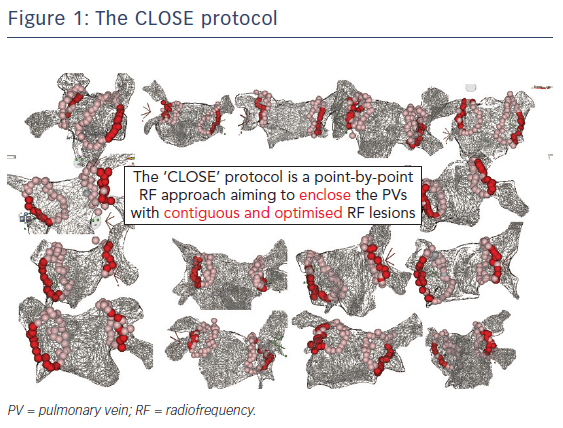
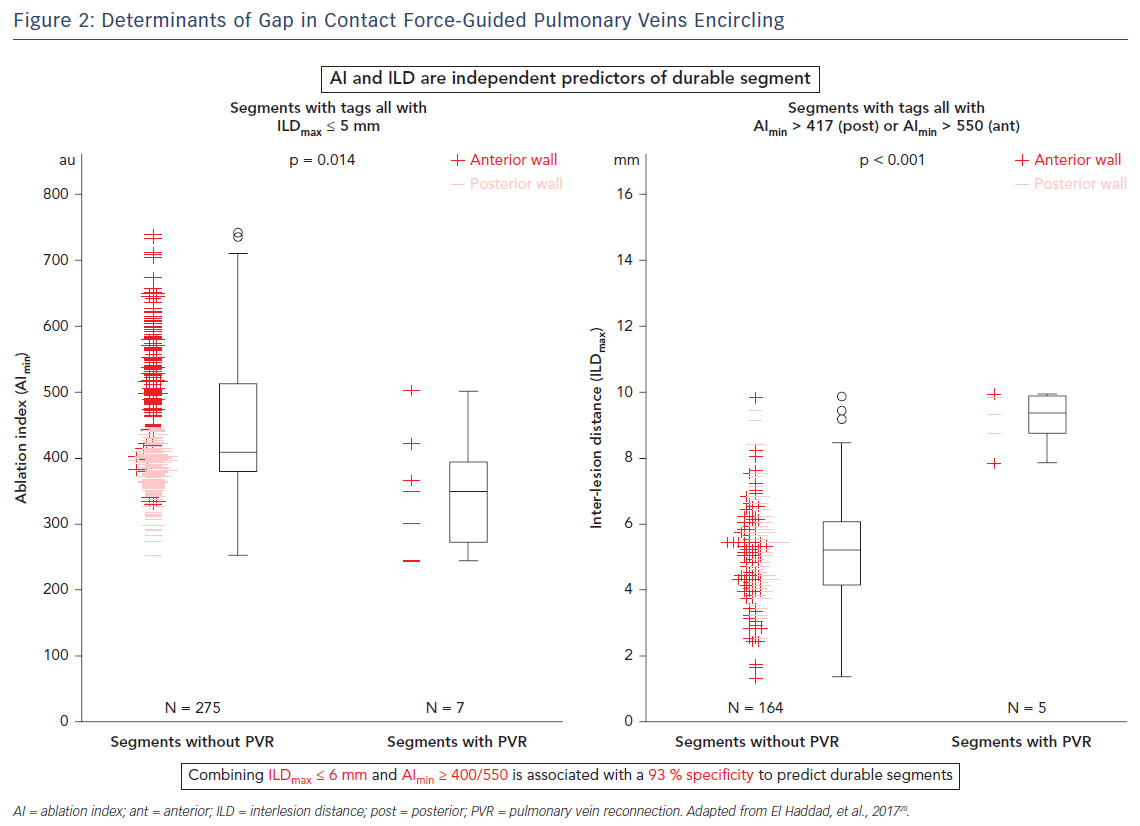
The CLOSE protocol is a point-by-point approach aiming to enclose PVs with contiguous and optimised RF lesions (Figure 1). This protocol was based upon a prior study from El Haddad et al.20 In this study 42 CF-guided PVI procedures were analysed with the aim of identifying determinants of acute and late gaps after CF-guided PV encircling. Each circle was subdivided into 10 segments. For each segment, the weakest link in the circle was determined. Two independent predictors of durable segments were identified: ablation index (AI) and interlesion distance (ILD). This study suggests that gaps are determined by insufficient lesion depth and/or discontiguity within the deployed RF circle. Vice versa, combining ILDmax ≤6 mm and AImin ≥400 (posterior)/550 (anterior) was associated with a 93 % specificity to predict durable segments (Figure 2).20
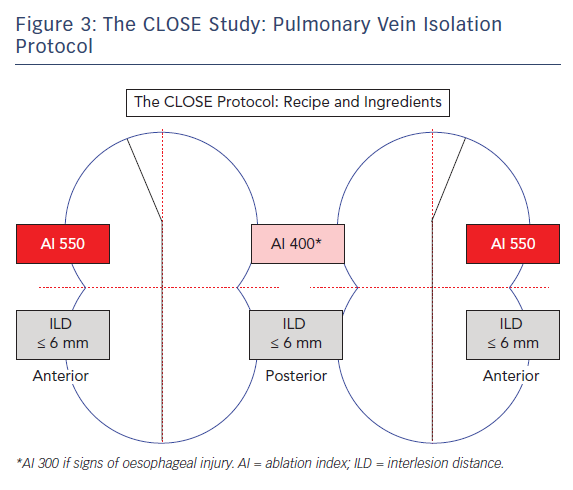
It was therefore hypothesised that an RF strategy aiming to enclose the PVs with strict criteria for lesion depth and ILD would lead to durable PV isolation.21 This CLOSE protocol was evaluated in >400 patients with PAF. The protocol involved creating one prespecified circle enclosing the veins. This was nephroid-shaped (towards the carina) (Figure 3) and 10–12 cm at the perimeter per circle. Ablation was performed where the atrial myocardium connects to the PV sleeve, and typically with a >2 cm distance between the ablated anterior and posterior carina. Lesions were closely spaced: an ILDmax ≤6 mm, based on previous study findings20,22,23 and energy (35 W) was delivered to reach a minimal AI ≥400 at the posterior wall and ≥550 at the anterior wall. The AI target was reduced to 300 (corresponding to an application of only 11–12 seconds) in the event of signs of oesophageal injury (pain during local anaesthesia or temperature probe heating during general anaesthesia). The procedural endpoint was not PVI per se but a perfect circle leading invariably to PVI.
More specifically, the CLOSE protocol is performed using the ThermoCool SMARTTOUCH and LASSO® catheter (Biosense Webster). Before RF was undertaken, the circle was pre-designed around the veins based on anatomy, CF, local electrograms and position of the LASSO catheter. Using the CARTO VISITAG Module and respiratory gating, the lesion marker settings were based upon stability (3 mm for 8 seconds) and force (30 % >4 g). Procedures were carried out under sedation or general anaesthesia. A point-by-point contiguous line was created to enclose the PVs. During RF, a size tag 3 mm and real-time ruler were employed. Power settings were as high as reasonably achievable with a maximum of 35 W (30 cc cooling). Following ipsilateral encircling, ILD and AI criteria were systematically verified. The ultimate goal was enclosing the circle with the exact required quantity of RF tags, around 30 per circle (corresponding to an RF time of approximately 15 minutes).
Recently, procedural- and 1-year outcome in 130 patients with PAF were reported by Taghji et al.21 An unprecedented high rate of acute durable PV isolation (98 % adenosine-proof isolation) was paralleled by excellent 1-year outcome. At 12 months, freedom from documented AF/ atrial tachycardia (AT)/ atrial flutter (AFL) was reported in 92.3 % of all patients.21 Recurrence was defined as any AF, AT or AFL >30 seconds on Holter ECGs at 3, 6 and 12 months. Subgroup analysis showed that freedom from documented AF/AT/AFL in patients off antiarrhythmic drug therapy (n=104) was 91.3 %; freedom from documented AF/AT/AFL in patients on antiarrhythmic drug therapy (n=26) was 96.2 %.
Among 235 patients in a safety cohort (130 in the pilot study and 105 in a follow-up study) complications were reported in only three cases (1.2 %). These comprised one transient ischaemic attack-like event (0.42 %), one symptomatic PV stenosis (operator error; 0.42 %) and one femoral artery pseudo aneurysm requiring surgery. No incidences of steam pops, death, stroke or tamponade were reported.
Several other studies evaluating the efficiency, safety and efficacy of CLOSE are ongoing. The ongoing Evaluation of Ablation Index and VISITAG Use for PVI in Patients with PAF (VISTAX) study aims to evaluate an AI- and ILD-guided enclosing strategy in over 300 PAF patients. The study began early in 2017 and is being performed in 19 European centres. The CLOSE-Guided Pulmonary Vein Isolation as Cure for Paroxysmal Atrial Fibrillation? (CLOSE to CURE) study is a single-centre, patient-controlled trial evaluating CLOSE-guided PVI in 105 PAF patients.24 A subcutaneous loop recorder monitors the patients 2 months before until 3 years after PVI. The last enrolment was in April 2017. The patients are not taking antiarrhythmic drug therapy and oral anticoagulants are stopped when there is absence of arrhythmia, low stroke risk and absence of atrial scar.
In summary, an ablation strategy guided by the CARTO VISITAG Module with Ablation Index and respecting strict criteria of lesion contiguity and lesion depth results in a significant improvement in acute PVI durability and a high single-centre-procedure, arrhythmia-free survival at 1 year (92.3 %). Because of the standardised workflow to reach an objective procedural endpoint, reproducible results are expected across centres and operators.
Conclusion
A growing body of evidence supports the efficacy and safety of CF-sensing technology. To optimise clinical outcomes in PVI, there is a need for a standard RF ablation procedure. Consistency and lesion contiguity are essential in such a procedure. An objective lesion assessment prediction tool such as CARTO VISITAG Module with Ablation Index is also important to achieve a significant improvement in acute PVI durability and a high rate of arrhythmia-free survival at 1 year (92.3 %). The use of a standardised workflow is expected to increase the reproducibility of results across centres and operators, and to increase efficiency of PVI procedure. It should be emphasised that the current approach is limited to only paroxysmal, not necessarily persistent, AF beyond PVI.
The next challenge in PV ablation is to maintain this high durability while avoiding overshoot. Only then will the unique advantage of PV ablation be exploited to its full potential.