Ventricular tachycardia (VT) is a significant cause of morbidity and mortality in patients with structural heart disease (SHD). While implantable cardioverter-defibrillators (ICDs) have been shown to be effective in preventing sudden death due to ventricular arrhythmias, they are not able to prevent recurrent VT episodes. Antiarrhythmic drugs (AADs) have some demonstrated efficacy in preventing VT episodes, although options remain limited in patients with SHD and the degree of benefit is suboptimal. Amiodarone is the most effective AAD, but is associated with significant side-effects with long-term use, and many patients are unable to tolerate the medication.
With the advances in technology over the past two decades, catheter ablation has become an increasingly utilised adjunctive treatment modality for patients with VT. Catheter ablation has been clearly shown to be effective in decreasing the number of VT episodes, including antitachycardia pacing (ATP) therapies and shocks. While catheter ablation reduces long-term VT recurrences, it has not been shown to provide mortality benefit in patients with SHD.1 In this regard, patients still tend to be referred for ablation late in their disease course. Two prior studies have shown that early referral for ablation in patients with ischaemic and non-ischaemic cardiomyopathy (ICM and NICM) is associated with improved long-term VT suppression.2,3
There have been limited data published from prospective randomised controlled trials examining the long-term outcomes of VT ablation. Most outcome studies have been single or multicentre retrospective observational experiences, and case series with limited sample sizes, so are subject to a variety of biases and confounding factors.
In this article, we will summarise the available data on long-term outcomes following VT ablation in patients with different types of SHD.
Heterogeneity of Ventricular Tachycardia Ablation Studies – Impact on Long-term Outcomes
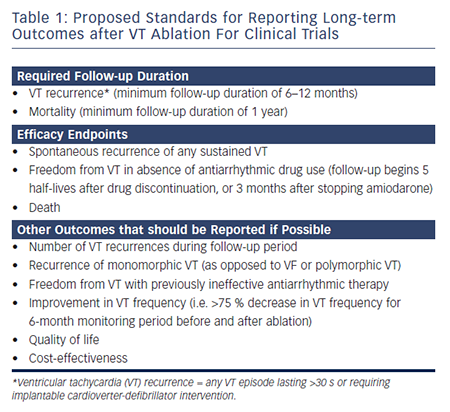
The 2009 VT ablation guidelines have proposed standards for reporting long-term outcomes after VT ablation for clinical trials (see Table 1).4 However, the outcomes in previous smaller retrospective studies have been quite variable. The patient populations in different studies may vary significantly with regards to the number of VT episodes, haemodynamic stability of VTs, presence of back-up ICD, etc. Additionally, ablation strategies (i.e. endocardial versus endocardial/epicardial approach; mapping and ablation approaches) may differ between studies, based on investigator and institutional preferences. Substrate-based ablation approaches, which are often used in patients with haemodynamically unstable VT, may differ greatly between VT ablation centres (i.e. late potential ablation, local abnormal ventricular activity ablation, scar homogenisation, scar dechannelling, linear ablation strategies and core isolation).5–12 When VT recurs after an initial ablation procedure, repeat ablation may be necessary to achieve long-term suppression.13 While some studies report long-term outcomes following the index ablation procedure, others have referred to long-term outcomes following the last ablation procedure in patients requiring multiple procedures. Therefore, it is of utmost importantance that providers carefully review the methods of each study, particularly inclusion and exclusion criteria, prior to extrapolating results to individual patients in clinical practice.
Ischaemic Cardiomyopathy
There have been only three major prospective randomised clinical trials examining long-term outcomes after VT ablation in patients with ICM.14–16
The first large-scale prospective randomised controlled trial examining ablation versus medical therapy in patients with ICM was the Substrate Mapping and Ablation in Sinus Rhythm to Halt Ventricular Tachycardia (SMASH-VT) study.14 This trial initially enrolled only patients with recently implanted ICDs for secondary prevention and later included those who underwent ICD implantation for primary prevention who had received an appropriate ICD therapy for a single VT or ventricular fibrillation (VF) episode. A total of 128 patients were enrolled and randomly assigned to catheter ablation or medical therapy (64 in each arm). The primary endpoint was survival from any appropriate ICD therapy (ATP or shock), and secondary endpoints included freedom from inappropriate ICD shock, death and ICD storm (≥3 shocks in 24-hour period). Freedom from recurrent VT/VF resulting in appropriate ICD therapy after 2 years of follow-up was significantly higher in the ablation arm (88 versus 67 %; HR 0.35; 95 % CI 0.15–0.78; p=0.007) compared with controls.14
The second landmark trial was the Ventricular Tachycardia Ablation in Coronary Heart Disease (VTACH) study: a prospective, open, randomised control trial involving 16 centres in four European countries.15 The investigators enrolled 110 patients with haemodynamically stable VT, prior MI and reduced left ventricular ejection fraction (LVEF), who were randomly assigned to catheter ablation and ICD versus ICD alone. The primary endpoint was time to first VT/VF recurrence. Of the 107 patients included in the analysis (52 ablation; 55 control), median time to VT/VF recurrence in the ablation group was longer than the control group (18.6 versus 5.9 months). After 2 years, those randomised to ablation had superior VT/VF-free survival (47 versus 29 %; HR 0.61; 95 % CI 0.37–0.99; p=0.045) and were more likely to be free from cardiac hospital readmission (67 versus 45 %; HR 0.55; 95 % CI 0.30–0.99; p=0.044). Additionally, those in the ablation group on average had significantly fewer appropriate ICD shocks per patient year (mean 0.6±2.1 versus 3.4±9.2 shocks; p=0.018).15
The Catheter Ablation for VT in Patients with Implantable Cardioverter Defibrillator (CALYPSO) trial was a small prospective randomised trial comparing a strategy of early catheter ablation versus AADs for VT in patients with ICM with ICDs who had received appropriate ICD therapies for VT.16 This multicentre pilot study enrolled a total of 27 patients (13 ablation; 14 AAD), only 17 (71 %) of whom completed 6 months of follow-up. Rates of VT recurrence in this population of patients with multiple prior appropriate ICD therapies were significantly higher than those previously reported in SMASH-VT and VTACH: 62 % had recurrent VT in the ablation arm at 6 months following the ablation procedure.16
The 2-year VT-free survival rates in SMASH-VT and VTACH were 88 % and 47 %, respectively, while the 6-month VT-free survival rate in the much smaller CALYPSO trial was significantly lower (38 %), probably due to the fact that the trial selectively enrolled higher-risk patients known to have recurrent VT requiring appropriate ICD therapies.
Prospective non-randomised studies examining patients with ICM and varying VT burden have reported a wide range of long-term VT-free survival following ablation.
In 1997, Strickberger et al. prospectively enrolled 21 patients with ICD and frequent ICD therapies (mean of 25±88 ICD therapies within 36±51 days preceding ablation). Acute abolition of the clinical VT occurred in 76 %.17 Although this study did not report overall long-term VT-free survival, they did report a decrease in frequency of ICD therapies and improvement in quality of life in those with acute ablation success over a follow-up period of 11.8±10 months.17
The Cooled RF Ablation System clinical trial (2000) was a prospective, observational trial that included 146 patients with SHD, the majority (82 %) of whom had ICM.18 All patients had ICD implanted with haemodynamically stable VT and had failed at least two AADs. Mean follow-up duration was 243±153 days. One year after ablation, the VT recurrence rate was 56 %, while the 1-year mortality rate was 25 %. Of the 122 patients with at least 2 months clinical follow-up, 81 % had >75 % reduction in VT frequency in the first 2 months after ablation. The Multicenter Thermocool VT Ablation Trial was a prospective observational trial that enrolled 231 patients with recurrent monomorphic VT in setting of ICM treated with catheter ablation.19 This study was focused on evaluating the efficacy of VT ablation in very high-risk patients, and therefore included patients with haemodynamically unstable, unmappable and multiple VTs. After 6 months, 53 % of patients were free from recurrent incessant VT or intermittent VT and the mortality rate at 1 year was 18 %.19
The EURO-VT study was a multicentre, prospective, non-randomised observational study that enrolled 63 ICM patients with multiple (>4) episodes of symptomatic VT within 6 months (or VT storm) of randomisation.20 Over mean follow-up of 12 months, 49 % had VT recurrence, although the majority (79 %) of those with VT recurrence had reduction in ICD therapies.20
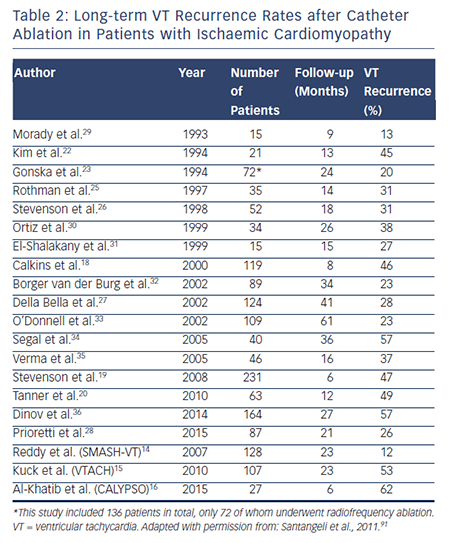
Retrospective observational studies examining VT ablation for patients with ICM published have reported varying long-term rates of VT recurrence ranging from 16 to 66 %. Table 2 summarises long-term outcomes of VT ablation in patients with ICM.10,14–16,18–36
Non-ischaemic Cardiomyopathy
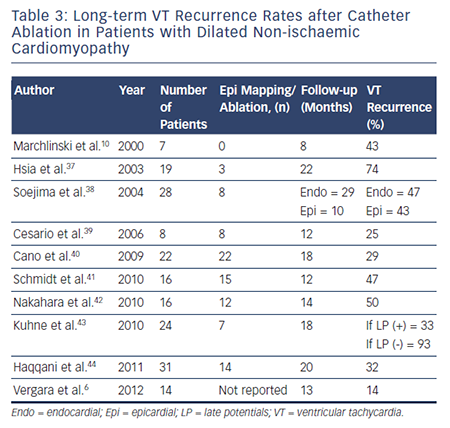
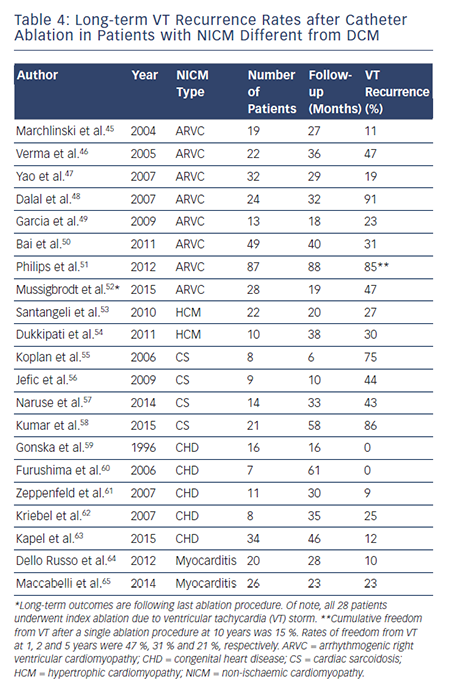
To date, there have been no prospective randomised trials describing outcomes following VT ablation in patients with NICM. Patients with NICM have higher rates of acute procedural failure and long-term VT recurrence following ablation compared with ICM.28,36 Unlike ICM, where the underlying substrate is relatively homogeneous, patients with NICM have heterogeneous substrates that reflect the variety of pathogenetic processes. However, the distribution of abnormal substrates in patients with non-ischaemic pathology has been shown fairly homogeneous, with a typical involvement of perivalvular regions and high prevalence of intramural and/or epicardial substrates. Table 3 summarises long-term outcomes of VT ablation in dilated NICM (DCM),6,10,37–44 and Table 4 summarises long-term outcomes of VT ablation in other forms of NICM.45–65
The Heart Center of Leipzig VT (HELP-VT) study was a prospective observational European single-centre study that enrolled 63 patients with NICM and 164 patients with ICM who were treated with VT ablation between 2008 and 2011.36 Activation mapping and ablation were performed in nearly half of patients, and substrate modification was not uniformly performed. Acute procedural success (defined as complete non-inducibility after ablation) was achieved in 66.7 % of those with NICM (versus 77.4 % in ICM; p=0.125). Long-term VT-free survival was significantly lower for NICM compared with ICM: cumulative VT-free survival after median follow-up periods of 20 and 27 months for NICM and ICM, respectively, were 23 % and 43 % (HR 1.62, 95 % CI 1.12–2.34; p=0.01). VT-free survival rates at 1 year were 40.5 % for NICM and 57 % for ICM.36
Proietti et al. recently reported their experience with a substrate-guided ablation approach in 55 NICM and 87 ICM patients.28 They showed lower rates of freedom from recurrent VT in those with NICM compared with ICM (49 % versus 74 %; p=0.03) over a follow-up period of 21.1 months. They attributed their higher rates of long-term success (compared with previous reports) to the fact that higher rates of acute procedural success might have been achieved using a substrate-based approach.28
One large single-centre retrospective observational study, which examined 226 patients with NICM treated with VT ablation, reported 29 % rate of death or transplant at long-term follow-up (4.4±3.3 years follow-up), while the secondary composite endpoint of death, heart transplantation or hospitalisation for VT recurrence at 1 year (after the last ablation) was 31 %.66
Dilated Cardiomyopathy
Patients with DCM tend to have worse prognosis after VT ablation compared with arrhythmogenic right ventricular cardiomyopathy (ARVC) and congenital heart disease (CHD).66 Retrospective studies have demonstrated VT recurrence rates ranging between 46 and 61 % during long-term follow-up (mean 11–25 months for individual studies).38
Patterns of scar in non-ischaemic DCM have been previously described. Classically, there is involvement of the base of the heart along the perivalvular region, particularly around the basolateral LV.37,67 In this group of patients, the prevalence of intramural and epicardial substrates is high. At our institution, up to 11.3 % of all NICM patients had an isolated septal substrate. Haqqani et al. reported that this particular population frequently required multiple procedures to achieve VT control. VT recurred in 32 % of patients over mean follow-up of 20 months after ablation.44 Oloriz et al. later classified 87 NICM patients treated with ablation as having either predominantly anteroseptal versus inferolateral scar based on endocardial unipolar voltage mapping.68 Patients with inferolateral scar frequently had epicardial substrate, while those with anteroseptal scar more often had intramural septal substrate. They noted a higher VT recurrence rate in those with anteroseptal scar (74 vs 25 %; p<0.001), resulting in higher redo ablation rate (59 versus 7 %; p<0.001). Anteroseptal scar was an independent predictor of VT recurrence in the multivariate analysis (HR 5.5; p<0.001).
Arrhythmogenic Right Ventricular Cardiomyopathy
Long-term success rates of VT ablation in patients with ARVC have historically been quite variable and highly dependent on the specific ablation approach adopted.45–48,52 Over a decade ago, Marchlinski et al. reported a long-term VT suppression rate of 89 % in 19 patients who underwent endocardial VT ablation over a mean follow-up duration of 27 months.45 They identified that in these patients, certain areas such as the perivalvular tricuspid/pulmonary valve regions and the RV free wall and septum (but not the RV apex) were more likely to harbour areas with abnormal electrograms. Verma et al. showed 1, 2, and 3-year VT recurrence rates of 23 %, 27 %, and 47 %, respectively.46 Dalal et al. later reported much worse long-term outcomes in 24 patients treated with endocardial VT ablation at 29 centres across the country between 1999 and 2006.48 In their study, they reported VT recurrence rates after a single procedure of 64 %, 75 %, and 91 % after 1, 2, and 3 years, respectively. They hypothesised that the dismal long-term success was due to the fact that ARVC was an electrically progressive disease.48 Later, Riley et al. elegantly showed with serial electroanatomical voltage mapping that although the RV progressively dilates, only the minority of patients have progression of endocardial scar, suggesting that an aggressive substrate modification may be effective in long-term VT control.69
In fact, given the more extensive epicardial pathological substrate in ARVC, catheter ablation approaches using a combination of endoepicardial substrate based ablation have been recently shown to significantly improve VT-free survival at the short to mid-term followup. 49–51,70 Garcia et al. reported a series of 13 ARVC patients with VT undergoing endo-epicardial mapping and ablation.49 The authors confirmed a more extensive epicardial involvement in these patients, with a reported success rate of 77 % over a mean follow-up time of 18 months. Similar findings have been reported by Bai et al.50 in a multicentre series of 49 patients undergoing either endocardial-only ablation (n=23), or endo-epicardial ablation (n=26). After a follow-up of at least 3 years, VT-free survival achieved 52.2 % in the endocardial-only ablation group and 84.6 % in the endo-epicardial ablation group. Two other studies from different institutions across US and Europe have recently reported the same results.51,70 In conclusion, endo-epicardial ablation is significantly more effective that endocardial-only procedures in achieving VT-free survival in patients with ARVC, essentially due to the peculiar epicardial to endocardial progression of the disease.
Hypertrophic Cardiomyopathy
Malignant ventricular arrhythmias in patients with hypertrophic cardiomyopathy (HCM) stem from pathological myocardial fibrosis, disruption of cellular architecture and hypertrophied myocytes,71,72 which constitute the substrate for reentrant VTs. Studies evaluating the pattern of myocardial tissue scarring in HCM reported a high prevalence of mid-myocardial and epicardial fibrotic areas.73,74 Remarkably, in a small subset of patients in whom the disease evolves to the end-stage leading to aneurysm formation, a transmural scar can be detected.75,76 Until recently, the clinical experience with catheter ablation of VT was limited to patients with end-stage forms of the disease with apical aneurysms.76,77 Two recent studies have reported the feasibility and safety of VT ablation in larger series of patients with HCM also without apical aneurysms.53,54 In a multi-centre observational study, Santangeli et al. evaluated the role of VT ablation in a series of 22 patients with multiple episodes of drug-refractory VTs.53 In this study, an endo-epicardial ablation was required in 59 % of cases, and no major procedural complication was observed. After an average follow-up of 20 months, freedom from recurrent VT reached 73 %. In a subsequent study, Dukkipati et al. reported 10 patients with HCM-related monomorphic VT treated with combined endocardial and epicardial ablation.54 Epicardial scar was identified in 80 % of patients, endocardial scar in 60 % and no scar in 10 %. Five patients had stable inducible monomorphic VT and were treated with combined endocardial and epicardial ablation. Four underwent combined endocardial and epicardial ablation with a substrate-based approach based on sites of late/fractionated potentials with good pace maps. The final patient was non-inducible and had no endocardial or epicardial scar, so no ablation was performed. During mean long-term follow-up of mean 37.4±16.9 months, only three (30 %) patients had ICD shocks for recurrent VT (including the lone patient who was noninducible and had no electroanatomic scar).54
Cardiac Sarcoidosis
From a clinical standpoint, cardiac sarcoidosis (CS) may be difficult to differentiate from other forms of NICM, such as ARVC.78 However, patients with CS typically present with more-extensive LV scar and may have septal involvement (which is rare in ARVC), in addition to worse overall long-term ablation outcomes.58,79
In 2006, Koplan et al. reported a 75 % VT recurrence rate within 6 months of ablation in eight patients with CS treated with catheter ablation for incessant VT.55 Two small observational studies showed long-term VT recurrence rates of 43–44 % over median follow-up periods of 10 and 33 months after ablation.56,57 In another study of eight patients with CS, the clinical VTs were successfully abolished in five (63 %).79 While the authors did not specify the recurrence rate of those with failed ablation, only one (20 %) of the five patients with successful ablation had recurrent VT after 6 months of follow-up.79
The largest study of catheter ablation for VT in patients with CS included 21 patients, who tended to have multiple inducible VTs, which were consistent with scar-related reentry.58 Voltage mapping demonstrated confluent RV and patchy LV scarring with a predilection for the septum, anterior wall and perivalvular regions. While RV epicardial scar is usually overlaid RV endocardial scar, this was not the case with the LV. The rate of complete acute procedural success was relatively poor, and freedom from VT after 1 year after a single procedure was only 25 % (37 % after multiple procedures). Ablation was effective, however, in acutely terminating VT storm in seven (78 %) of the nine patients who were referred for incessant VT.58
Repaired Congenital Heart Disease
Compared with other aetiologies of NICM, long-term outcomes in patients with repaired CHD are quite favourable.66 Smaller reports have shown rates of VT-free survival ranging between 75 and 100 % over long-term follow-up (mean follow-up durations ranging between 16–61 months).59–62 In the largest series to date, Kapel et al. reported outcomes in 34 patients with repaired CHD (82 % with repaired Tetralogy of Fallot) treated with catheter ablation targeting anatomic isthmuses containing reentrant VT circuits.63 During long-term followup (mean 46 months), VT recurred in only 11.7 % of cases (0 % in those with complete procedural success versus 44 % in those without complete success), and one patient with poor cardiac function received an ICD shock for VF after ablation.63
Viral Myocarditis
Arrhythmias including VT often occur during the acute phase of viral myocarditis due to the presence of active inflammation. Later, the long-term sequelae of viral myocarditis including fibrosis and scar may predispose to reentrant VT. Imaging with MRI in patients with viral myocarditis has shown that different viruses tend to have different patterns of myocardial involvement.80 Due to the variability of the scar distribution, pre-procedural imaging (MRI or CT) may be helpful when performing VT ablation in these patients. Using an imaging-guided approach, Maccabelli et al. found that patients with myocarditisrelated VT very frequently have epicardial substrate.65 Long-term (median 23 months) freedom from recurrent VT after ablation in their cohort was 77 %. Dello Russo et al. subsequently studied 20 consecutive patients with biopsy-proven viral myocarditis and VT refractory to AADs referred for catheter ablation.64 During long-term follow-up (median 28 months), 90 % of the patients remained free of sustained VT and only two (10 %) patients died from non-arrhythmic cardiac causes.64
Ongoing Trials
There are multiple major ongoing trials that will further examine the long-term outcomes of VT ablation, and others which have been terminated due to difficult enrolment. Four of these ongoing trials include VT ablation versus Enhanced Drug Therapy (VANISH), the Substrate Targeted Ablation Using the FlexAbility Ablation Catheter System for the Reduction of Ventricular Tachycardia (STAR-VT) trial, PARTITA and the BERLIN study. Additionally, several ongoing studies are analysing effects of sympathetic modulation, including bilateral cardiac sympathetectomy (Cardiac Denervation Surgery for Prevention of Ventricular Tacharrhythmias [PREVENT VT])81 and renal sympathetic denervation (Renal SympathetiC Denervation to Suppress Ventricular Tachyarrhythmias [RESCUE-VT] and Renal Sympathetic Denervation as an Adjunct to Catheter-based VT Ablation [RESET-VT])82,83 as adjunct measures to prevent recurrent VT.
The VANISH trial is a prospective observational trial that is aiming to compare ablation versus aggressive AAD therapy in patients with prior MI who present with recurrent VT.84 Included patients will have prior MI with ICD in place, and must have been treated with at least one appropriate ICD therapy, and have failed at least one AAD. Goal enrolment is 260 patients, and patients will be randomised to either ablation or aggressive AAD therapy (high-dose amiodarone or addition of mexilitine). Duration of follow-up is 5 years, and primary outcome is a composite of appropriate ICD shocks, VT storm and death. Secondary outcome is all-cause mortality. The trial has finished enrolling patients and has an estimated study completion date of March 2016.
STAR-VT is an open-label, prospective randomised trial that aims to examine whether scar-based VT ablation results in superior outcomes compared with routine AAD therapy in patients with monomorphic VT in the setting of ICM or NICM.85 Goal enrolment is 1,453 patients, and inclusion criteria include implantation of a St Jude Medical ICD or cardiac resynchronisation therapy device, ≥1 documented monomorphic VT episode (either spontaneous or induced during electrophysiological study or non-invasive programmed stimulation). Patients will be randomised to either substrate-guided ablation using the FlexAbility Ablation Catheter versus routine drug therapy and followed for 1 year. The primary outcome measure will be freedom from any ICD shock (appropriate or inappropriate) for recurrent sustained VT (>30 s) in one year, and secondary outcome measures include number of cardiovascular-related hospitalisations and emergency room visits. This trial is currently enrolling patients and has an estimated study completion date of May 2021.
The third large ongoing trial is the PARTITA trial, which is a large multicentre European trial aiming to determine whether timing of VT ablation after appropriate ICD shock affects long-term prognosis.86 The estimated enrolment is 590 patients who have ICD for primary or secondary prevention. After enrolment, all patients will remain in Phase A until receiving appropriate ICD shock, at which point they enter Phase B in which they will be randomised to immediate VT ablation after appropriate shock versus waiting until VT storm. Followup duration in Phase B is 2 years, and the primary outcome measure in Phase B will be worsening heart failure hospitalisations or allcause mortality. Secondary outcome measures include cardiovascular mortality, electrical storm or VT recurrence during Phase B. This study is currently recruiting patients, and has an estimated study completion date of September 2018.
The BERLIN study is a prospective randomised controlled trial taking place in Germany, which is aiming to enrol 208 patients with prior MI and LVEF 30–50 % who have an ICD indication and documented VT.87 Patients are randomised to either early ablation (immediately following ICD implantation) versus late ablation (after third ICD shock). Primary endpoints include all-cause mortality and hospital admission secondary to cardiac causes, while the secondary endpoint is time to first ICD shock.
Complications
While catheter ablation is an effective treatment option in the management of VT, it is not without risk. A recent meta-analysis reported overall complication rates in 8–10 % of procedures.88 While the majority of complications are related to vascular access, more serious complications, such as stroke or transient ischaemic attack, pericardial effusion or cardiac tamponade and even death, may rarely occur. Furthermore, since VT ablation procedures may be prolonged and significant amounts of fluids may be given during the procedure (particularly when ablating with irrigated catheters), close monitoring of haemodynamic and fluid status is paramount. Acute periprocedural haemodynamic decompensation occurred in 11 % of patients undergoing VT ablation for scar-related VT in one series.89 As such, patients should be medically optimised prior to the ablation procedure, and prophylactic support with percutaneous LV assist devices may be beneficial to facilitate mapping and ablation in certain high-risk patients.90
Timing and Patient Selection
Since ICD shocks are associated with increased mortality and morbidity, VT ablation should be considered in all patients with SHD and recurrent VT refractory to at least one AAD. Retrospective studies have demonstrated improved VT-free survival with an early ablation approach,2,3 and several prospective clinical trials examining timing of VT ablation are currently ongoing, as described above. VT ablation has been shown to have similar safety and efficacy in elderly patients so older age alone should not be a deterrent.21 The potential risks and benefits must be considered in each particular patient prior to deciding whether to proceed with VT ablation. In all patients with VT who have an appropriate periprocedural risk and in whom VT ablation is likely to be successful, we recommend consideration of ablation early in the course of treatment, especially in those who wish to avoid or are intolerant of AADs.
Conclusions
While catheter ablation is an effective treatment option for VT suppression in patients with SHD, long-term VT suppression rates vary based on the underlying aetiology. Although prior studies have not demonstrated long-term mortality benefit, ablation is effective in reducing long-term VT recurrences, abolishing VT storm and preventing ICD shocks. The overall outcomes associated with catheter ablation are worse in patients with NICM compared with those with ischaemic substrates, which is likely the result of more complex substrates in NICM patients with a higher prevalence of intramural and/or epicardial substrates. In survivors of VT who do not yet have ICDs in place, an early ablation strategy in addition to ICD may reduce the incidence of future ICD therapies. Ongoing studies are further evaluating whether earlier catheter ablation of VT is associated with improved outcomes.