Atrial fibrillation (AF) is associated with a fivefold increased risk for stroke, a twofold increased risk for dementia, and a tripling of risk for heart failure,1,2,3 while AF genetic risk is strongly associated with cardioembolic stroke.4In the Framingham Heart Study the percentage of strokes attributable to AF increases steeply from 1.5 % at 50–59 years of age to 23.5 % at 80−89 years of age.5,6 In the Danish National Patient Registry, the 5-year risk of stroke for men aged 50 years with no risk factors was 1.1 %, and with AF alone without additional risk factors 2.5 %, with the great majority not being anticoagulated. In men aged 70 years, the corresponding risks were 4.8 % and 6.6 %.7 Approximately 24 % of all strokes are due to AF,3 and 10 % of ischaemic strokes are associated with AF first diagnosed at the time of stroke.8 Numbers of AF-related incident ischaemic strokes at age ≥80 years have trebled over the last 25 years, despite the introduction of anticoagulants, and are projected to treble again by 2050.9 In addition, extracranial systemic embolic events constitute 11.5 % of clinically recognised thromboembolic events in patients with AF, and are associated with a high morbidity and mortality, comparable to that of ischaemic stroke.10 AF is the main cause of coronary embolism, being independently associated with an increased risk of myocardial infarction, especially non-ST-elevation myocardial infarction (NSTEMI) in women.11,12
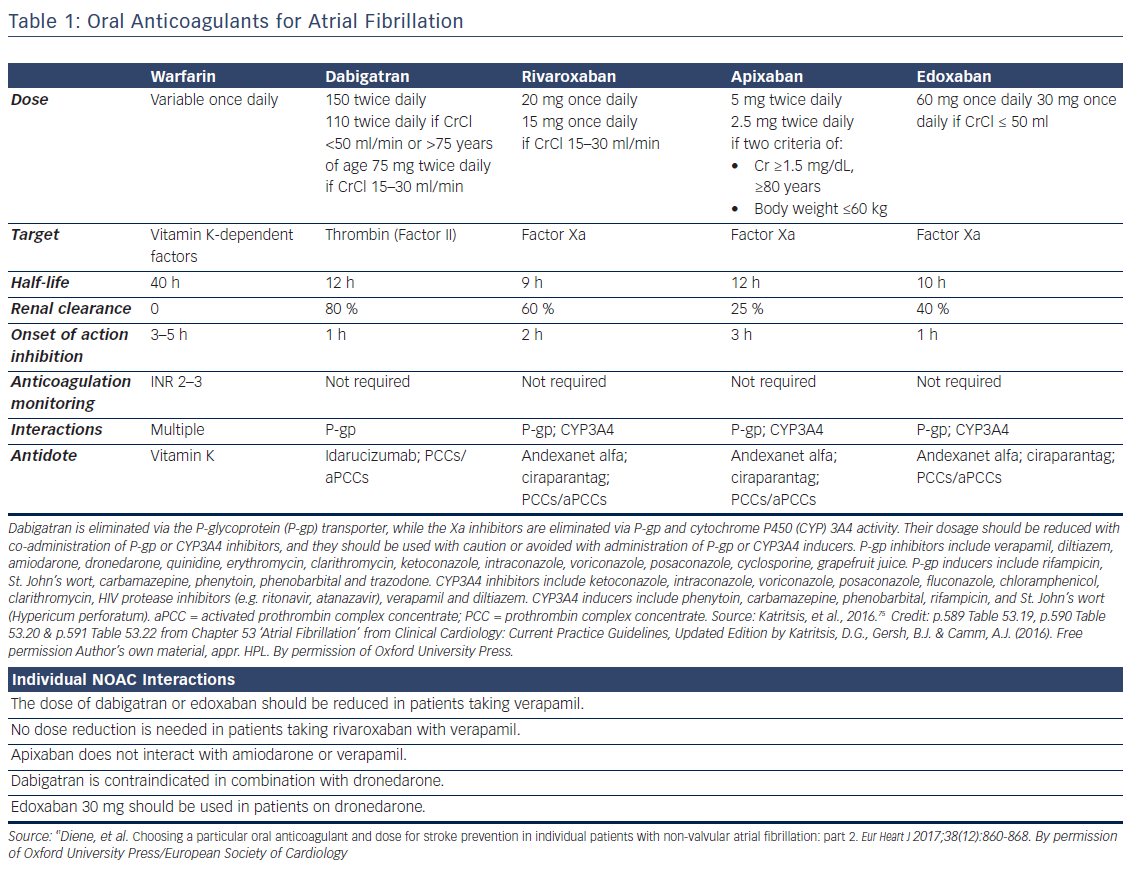
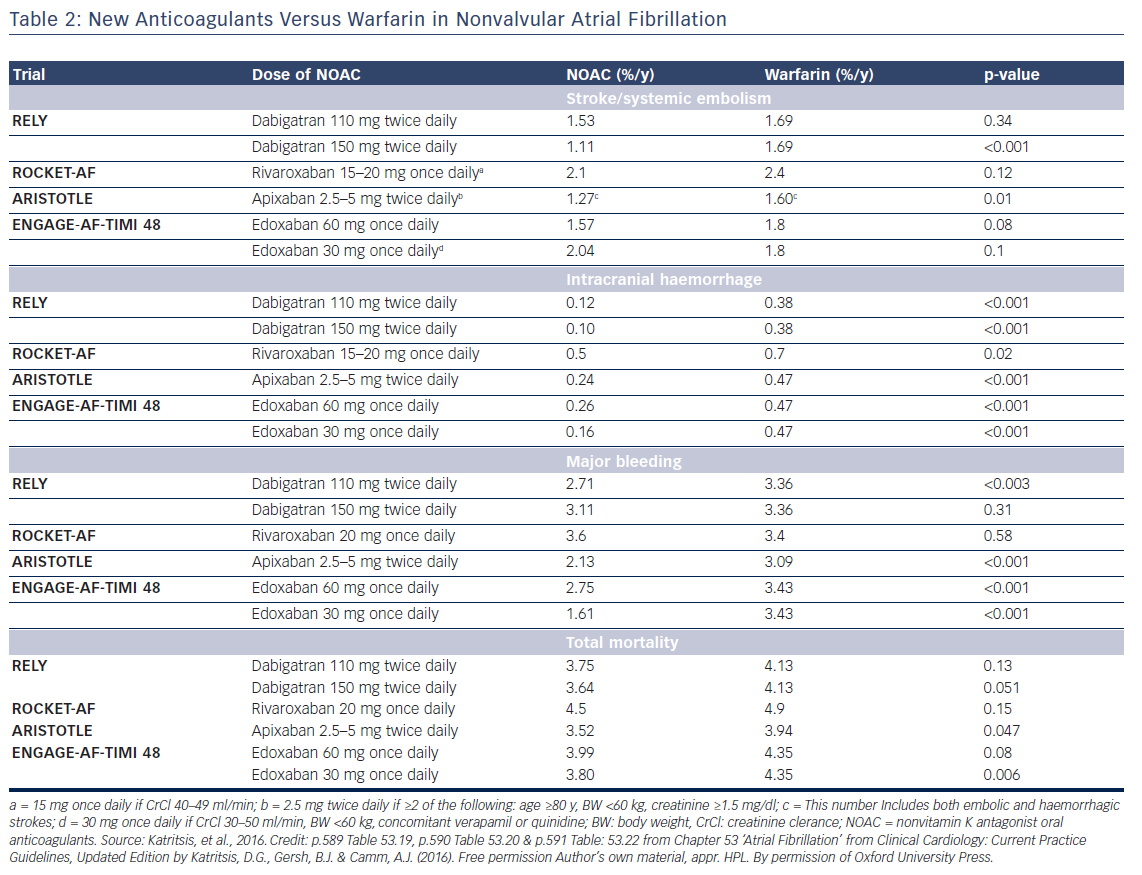
Oral anticoagulation, therefore, is mandatory for patients at high risk of thromboembolism as expressed by a CHA2DS2VASc score >2, but the risk of bleeding, assessed by various schemes such as the HAS-BLED, ATRIA, HEMORR2HAGES, and ORBIT,13,14 should also be taken into account. Low risk patients (score 0 for male and 1 for female) have a low risk of stroke (<1 % per year) and may be left without anticoagulation, since the benefit of anticoagulation does not outweigh the bleeding risk (net clinical benefit).15 Anticoagulation in patients with one stroke risk factor (CHA2DS2VASc score 1 for men and 2 in women) should be individualised since there is a significant increase in events rate in the presence of an additional risk factor.16–19 Direct oral anticoagulants (DOAC) are now recommended for non-valvular AF as a potential alternative to warfarin (see Tables 1 and 2).20
Assessment of Anticoagulant Effect and Antidotes of Specific Agents
Warfarin
The efficacy of the treatment with warfarin is directly related to the time in therapeutic range (TTR), that is, the percent time with international normalised ratio (INR) between 2.0 and 3.0. A target threshold TTR exists (estimated between 58 % and 65 %), below which there appears to be little benefit of oral anticoagulant (OAC) over antiplatelet therapy.21 The SAMe-TT2R2 [Sex (female); Age, 60 years; Medical history (more than two comorbidities); Treatment (interacting drug, e.g. Amiodarone); Tobacco use (doubled), and Race (doubled)] score is useful in identifying individuals who will not have good INR control (score ≥2).22
For excessive INR in the absence of bleeding, the American College of Chest Physicians (ACCP) guidelines recommend oral vitamin K (phytomenadione, 1–2.5 mg) only when INR is >10.23 IV vitamin K (1–2 mg) may also be given, although at 24 h oral vitamin K produces similar results. Oral dose is 2.5 mg or 1–2 mg of the IV preparation in a cup of orange juice. In the presence of major bleeding, four-factor prothrombin complex concentrate (4-PCC) is preferred to fresh frozen plasma since >1500 ml of fresh frozen plasma are needed to achieve a meaningful increase in coagulation factor levels.24 In a trial for reversal of VKA-associated major bleeding, the reported efficacy of 4-PCCs was 72 %, with 8 % thrombotic events, and 6 % mortality.25 The additional use of vitamin K 5 to 10 mg administered by slow IV injection is helpful in this setting.26
Dabigatran
Diluted thrombin time and ecarin clotting time or chromogenic assay are precise methods to assess the anticoagulant effect of dabigatran, but these methods are time-consuming and not widely available.27Activated partial thromboplastin time (aPTT) and prothrombin time (PT), measured in samples soon after the last dose, are prolonged by dabigatran but the correlation is not linear to guide dosage.28 However, in the presence of a normal aPTT, dabigatran is unlikely to contribute to bleeding, and aPTT can be used in emergencies as a rough estimate.29
Specific antidotes are under study.30,31 Idarucizumab, a monoclonal antibody fragment, completely reverses the anticoagulant effect of dabigatran within minutes and has been shown to be effective in initial clinical trials (2.5 g IV infusions no more than 15 min apart).32–34 In patients with acute major bleeding the reported efficacy was 71 %, with 10 % thrombotic events, and a mortality of 12 %.32 Idarucizumab for reversal of dabigatran was approved by the FDA in October 2015. Ciraparantag binds in a similar way to the new oral factor Xa inhibitors, and to dabigatran, but further clinical experience is needed.
Non-specific haemostatic agents are prothrombin complex concentrates (PCCs) and activated prothrombin complex concentrates (aPCCs). PCCs are plasma-derived products that contain 3 (factors II, IX, and X) or 4 (addition of factor VII) clotting factors in addition to variable amounts of heparin and the natural coagulation inhibitors protein C and protein S. aPCC contains mostly activated factor VII along with mainly non-activated factors II, IX, and X. Recombinant activated factor VII may also reverse the effect of non-vitamin K antagonist oral anticoagulants (NOAC) but increases the risk of thromboembolic effects by >1 %.31,35
In emergencies, gastric lavage in recent drug ingestion, haemodialysis, oral charcoal within 2 h following dabigatran ingestion, desmopressin, packed red cells in anaemia, platelet transfusions in patients receiving concurrent antiplatelet therapies, and fresh frozen plasma in the presence of dilutional coagulopathy or disseminated intravascular coagulation may also be tried as general measures.36 Prothrombin complex concentrates (PCCs and aPCCs) are more effective than fresh frozen plasma but they carry an absolute increase of thromboembolic events of 1 %.31
Factor Xa Inhibitors
Antifactor Xa assays may be used as an estimate of the anticoagulant effect.27 aPTT and PT are prolonged by Xa inhibitors, but cannot be used to guide dosage since the correlation is not linear.28 Diluted prothrombin time appears as the best test to use in emergency situations.29
Andexanet alfa, a recombinant protein that binds and sequesters factor Xa inhibitors has been successfully tried for apixaban and rivaroxaban (ANNEXA trials).37,38 It is given as 300 mg IV bolus that can be followed by an infusion of 4mg/min for 120 min. In patients with acute major bleeding the reported efficacy was 79 %, with 18 % thrombotic events, and a mortality of 15 % reported.38 Ciraparantag, (IV bolus of 100–300 mg), a synthetic molecule that binds specifically to unfractionated heparin and low-molecular-weight heparin, reversed edoxaban within 10–30 min,39 and is under study.
Xa inhibitors are not removed by dialysis, being protein bound. Gastric lavage in recent drug ingestion, and platelet and fresh frozen plasma transfusions may also been tried as general measures. As noted, PCCs and aPCCs are more effective but they carry an absolute increase of thromboembolic events of 1 %.31
Management of Stroke
Ischaemic Stroke
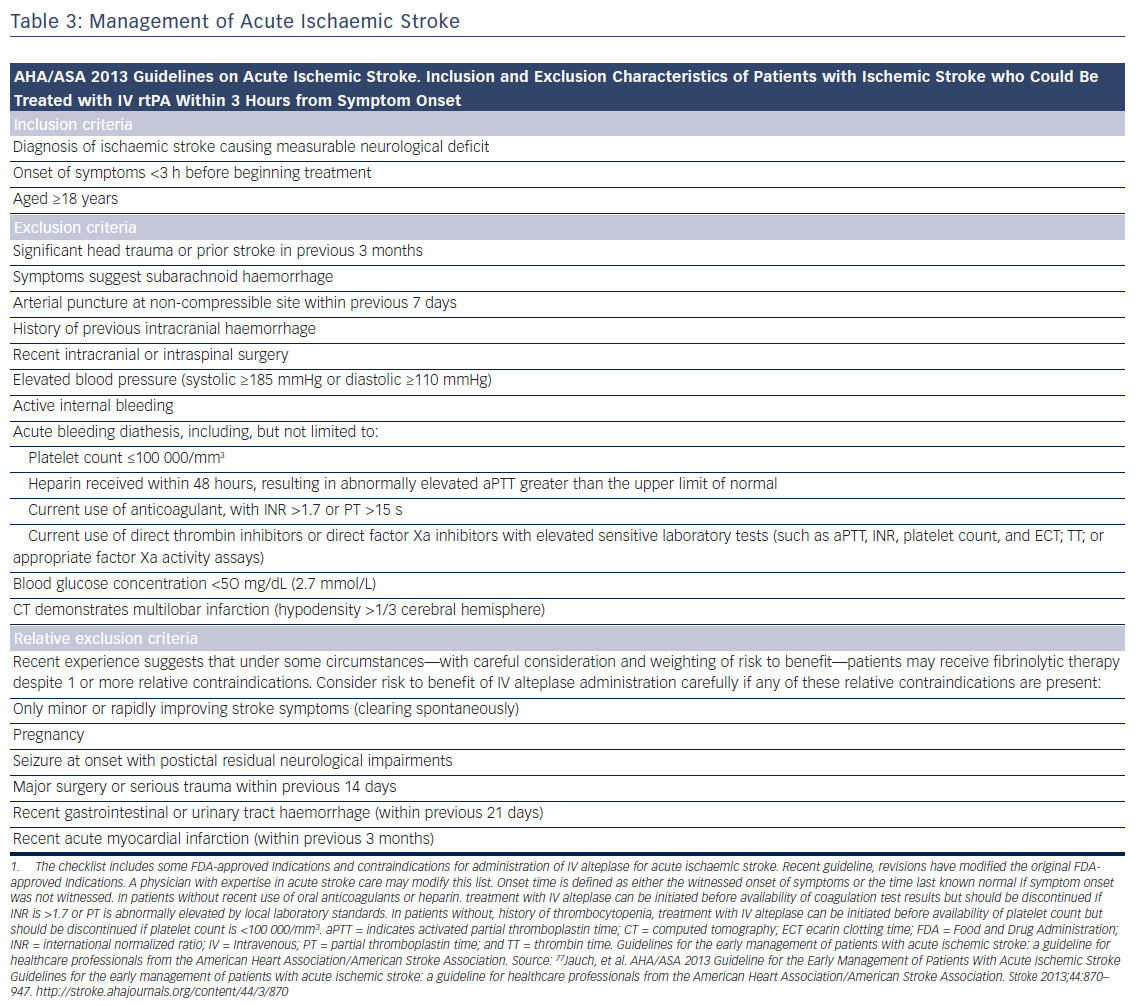
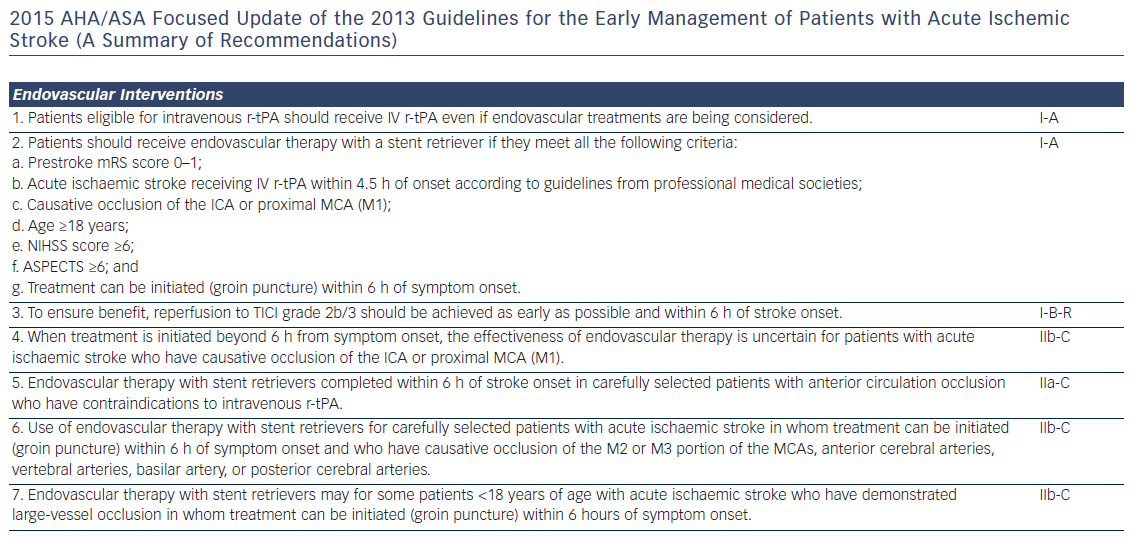
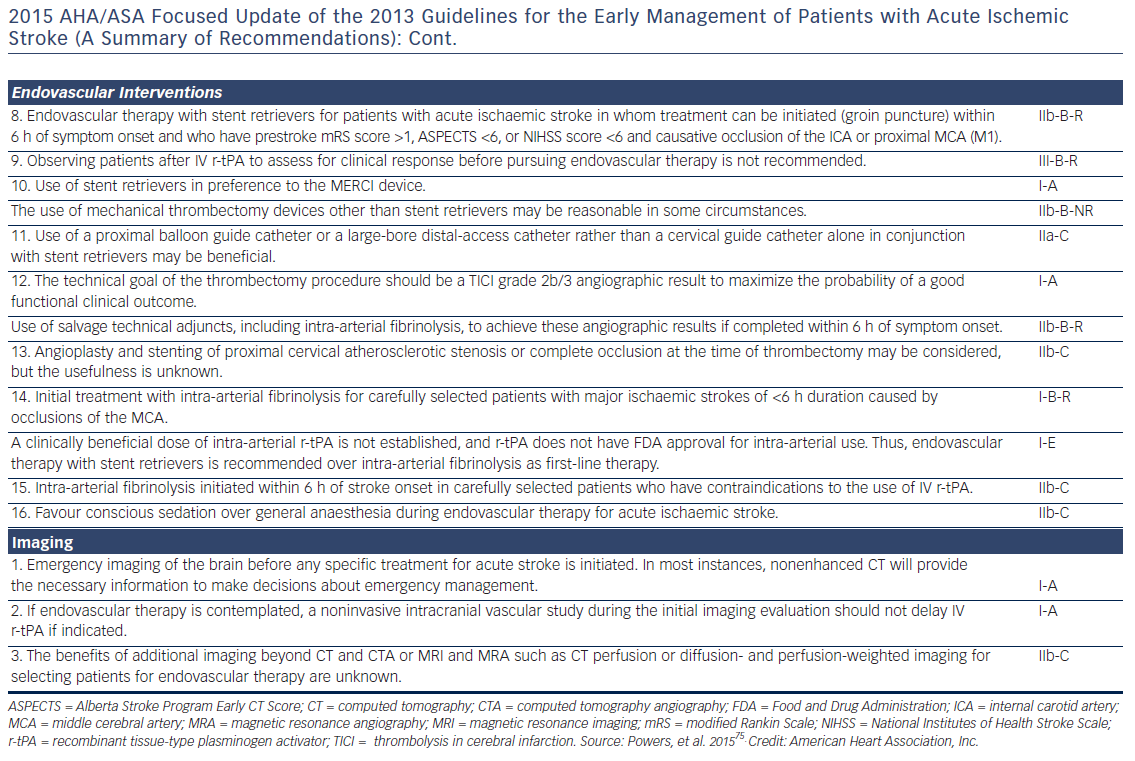
Patients presenting within 4.5 h after the onset of ischaemic stroke should be considered for IV rt-PA (0.9 mg/kg, with 10 % bolus, and the remainder over 60 min, maximum dose 90 mg). Diffusion-weighted magnetic resonance imaging and non-enhanced computed tomography are the most sensitive and specific methods for detecting ischaemic stroke and excluding intracerebral haemorrhage.40 They are necessary before intravenous rtPA to exclude intracranial haemorrhage (absolute contraindication) and to determine whether CT hypodensity of haemorrhage or MRI hyperintensity of ischaemia are present. Anticoagulation does not increase the risk of intracerebral haemorrhagic complications when INR is ≤1.7.41 In patients who present with stroke while taking new anticoagulants, if the aPTT is prolonged in a patient taking dabigatran or the prothrombin time with an Xa inhibitor, it should be assumed that the patient is anticoagulated, and thrombolysis should probably not be administered.40 However, in recent studies on patients with a ischaemic stroke and a NOAC taken within the last 48 h, thrombolysis with rt-PA or intra-arterial treatment had similar risk of symptomatic intracranial haemorrhage to that in patients on subtherapeutic VKA treatment (INR <1.7) or in those without previous anticoagulation.42,43 Criteria for fibrinolysis are presented in Table 3 and recommendations for secondary stroke prevention in Table 4 and Figure 1. Of note, age >80 years is not an exclusion criterion, provided it can be given within the first 3 h. Tenecteplase (0.25 mg/kg, administered as a single bolus, with a maximum dose of 25 mg), a more fibrin-selective agent, is superior to alteplase in patients subjected to fibrinolysis within 6 h after the onset of ischaemic stroke,44 but this was not verified in another comparison within 4.5 h after stroke.45 Anticoagulants and antiplatelet agents should be withheld the first 24 h following fibrinolysis. Labetalol (10–20 mg IV over 1–2 min, may repeat 1 time), or nicardipine (5 mg/h IV, titrate up by 2.5 mg/h every 5–15 min, maximum 15 mg/h) are recommended only when the blood pressure exceeds 180/110 mmHg.40 However, in patients who are not candidates for fibrinolysis, blood pressure lowering in acute stroke is not established to be useful with systolic blood pressure of 140 to 220 mmHg and without evidence of nonstroke end-organ damage, with the possible exception of an early (<6 h) BP lowering strategy.46 Thus, treatment of hypertension in this setting should be individualised. In patients who are candidates for fibrinolysis a pre-treatment BP <180/110 mmHg is mandatory.
Fibrinolysis offers recanalisation rate of <50 %, and large thrombi in vessels such as the distal internal carotid artery or the first segment of the middle cerebral artery respond poorly.47 Intra-arterial, catheter-based treatment administered within 6 h after acute ischaemic stroke using aspiration and stent retrievers has improved neurologic recovery, and reduced mortality compared to IV fibrinolysis, especially in the presence of a proximal cerebral arterial occlusion, and a small infarct or salvageable brain tissue on CT.48 It can be delivered with or without concomitant IV fibrinolysis, and preliminary data suggest that they might be useful up to 8 h49 or even 12 h50 after symptoms onset.
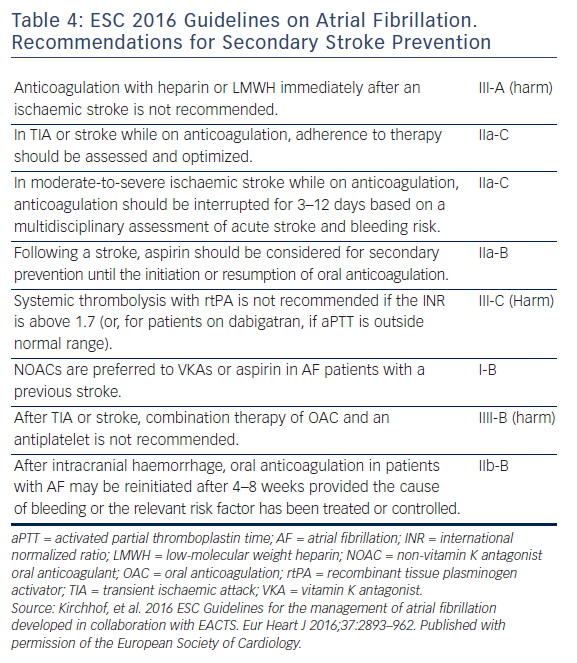
Re-initiation of anticoagulation following a non-fibrinolysed ischaemic stroke should be within 14 days after the onset of symptoms (AHA/ASA 2014 GL for prevention of stroke IIa-B), since the risk of early recurrence is as high as 8 %.50 In patients with a TIA, anticoagulation can be initiated 1 day after the onset of neurological symptoms, 3 days following small, non-disabling infarcts, 5–7 days following moderate infarcts, and 12–14 days following severe strokes.52,41 In the presence of high risk for haemorrhagic conversion (i.e. large infarct, haemorrhagic transformation on initial imaging, uncontrolled hypertension, or haemorrhage tendency), it is reasonable to delay initiation of oral anticoagulation beyond 14 days (AHA/ASA 2014 GL for prevention of stroke IIa-B).51 If anticoagulation is unsuitable or not feasible, dual antiplatelet therapy is recommended for secondary prevention (Clopidogrel With Aspirin in Acute Minor Stroke or Transient Ischemic Attack [CHANCE] trial).53 Since dabigatran 150 mg twice daily resulted in a significant reduction in both ischaemic and haemorrhagic stroke, should the acute ischaemic stroke occur while the patient is taking dabigatran 110 mg twice daily, or rivaroxaban or apixaban (neither of which significantly reduced ischaemic stroke compared with warfarin, in their respective trials), the use of dabigatran 150 mg twice daily instead, may be reasonable, but no direct data exist.54 Elective non-cardiac surgery may best be avoided for 9 months following a stroke, if possible.55
Intracerebral Haemorrhage
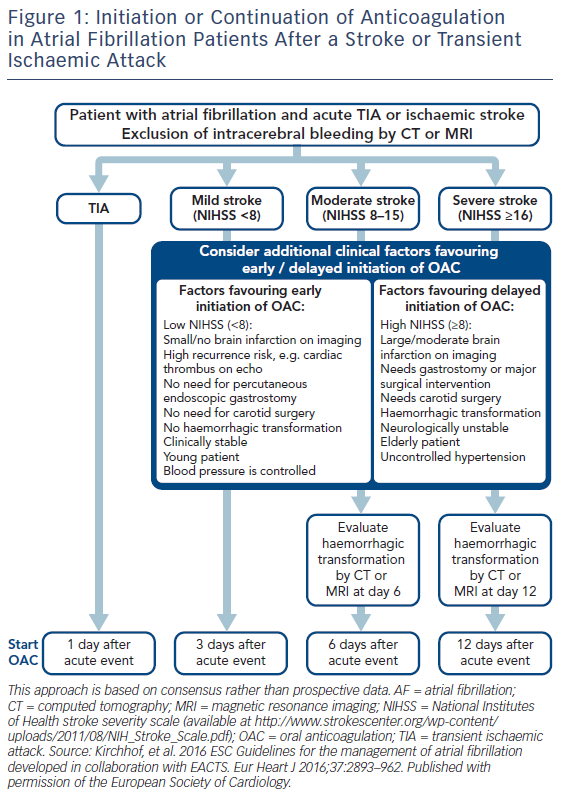
In the case of intracerebral haemorrhage, reversal of anticoagulation (INR <1.3) is needed with vitamin K 10 mg IV, to be repeated if the INR remains >1.4 at 24–48 h.56 If INR remains >1.4, four-factor PCCs are preferred to fresh frozen plasma. In patients receiving a DOAC, a specific antidote such as idarucizumab for dabigatran and andexanet alfa for Xa inhibitors are preferable, but if not available four-factor PCCs should be used. Protamine (1 mg for every 100 units of unfractionated heparin [UFH]) is used for unfractionated as well as for LMW heparin, and cryoprecipitate should be administered to patients who have received thrombolytics. Platelet transfusions are not recommended for patients who take antiplatelet agents, unless neurosurgical procedures are needed.56 Intensive treatment to lower the blood pressure with a target systolic level of <180 mmHg is recommended,57 but there has been evidence that values <160–140 mmHg may reduce haematoma enlargement and improve functional outcomes.58,59 After documentation of cessation of bleeding, low-dose heparin may be started 1–4 days from onset.57 The timing of resumption of oral anticoagulant is controversial (Figure 2). However, it may be started within 2 weeks since it is associated with a significant reduction in ischaemic stroke/all-cause mortality rates.60 DOAC are probably preferred if the haemorrhage happened on warfarin. In Asian patients, where the prevalence of intracranial haemorrhage is much higher than in non-Asians, warfarin was found beneficial following an event only in patients with CHA2DS2-VASc score ≥6.61
Management of Bleeding
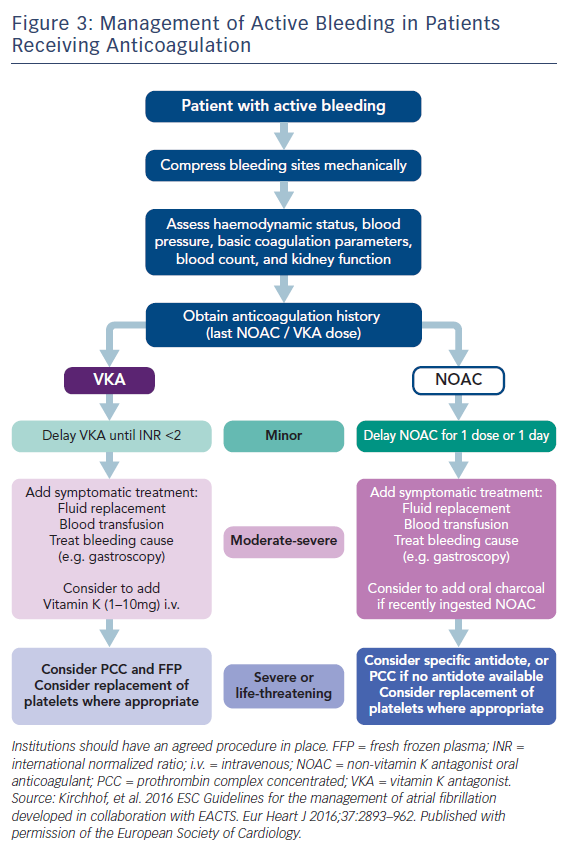
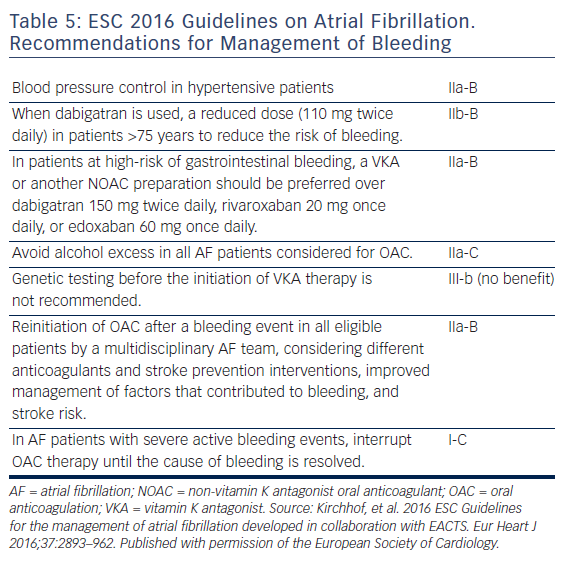
Antidotes and general measures as discussed, are summarised in Table 5 and Figure 3. Recent data suggest that anticoagulation should be restarted following discharge after an episode of GI bleeding.62 However, this study was too small for definitive conclusions.
Perioperative Anticoagulation
Warfarin
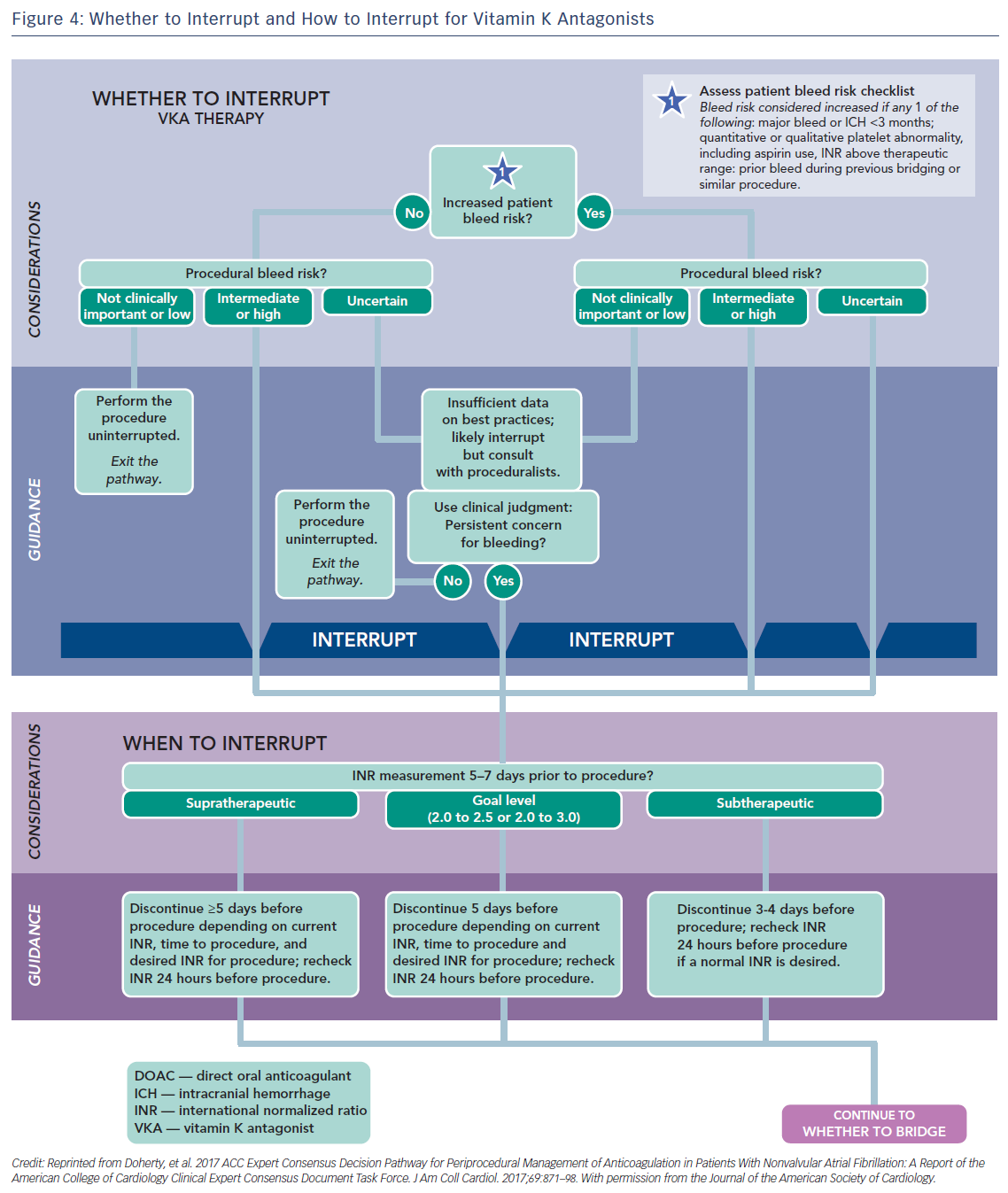
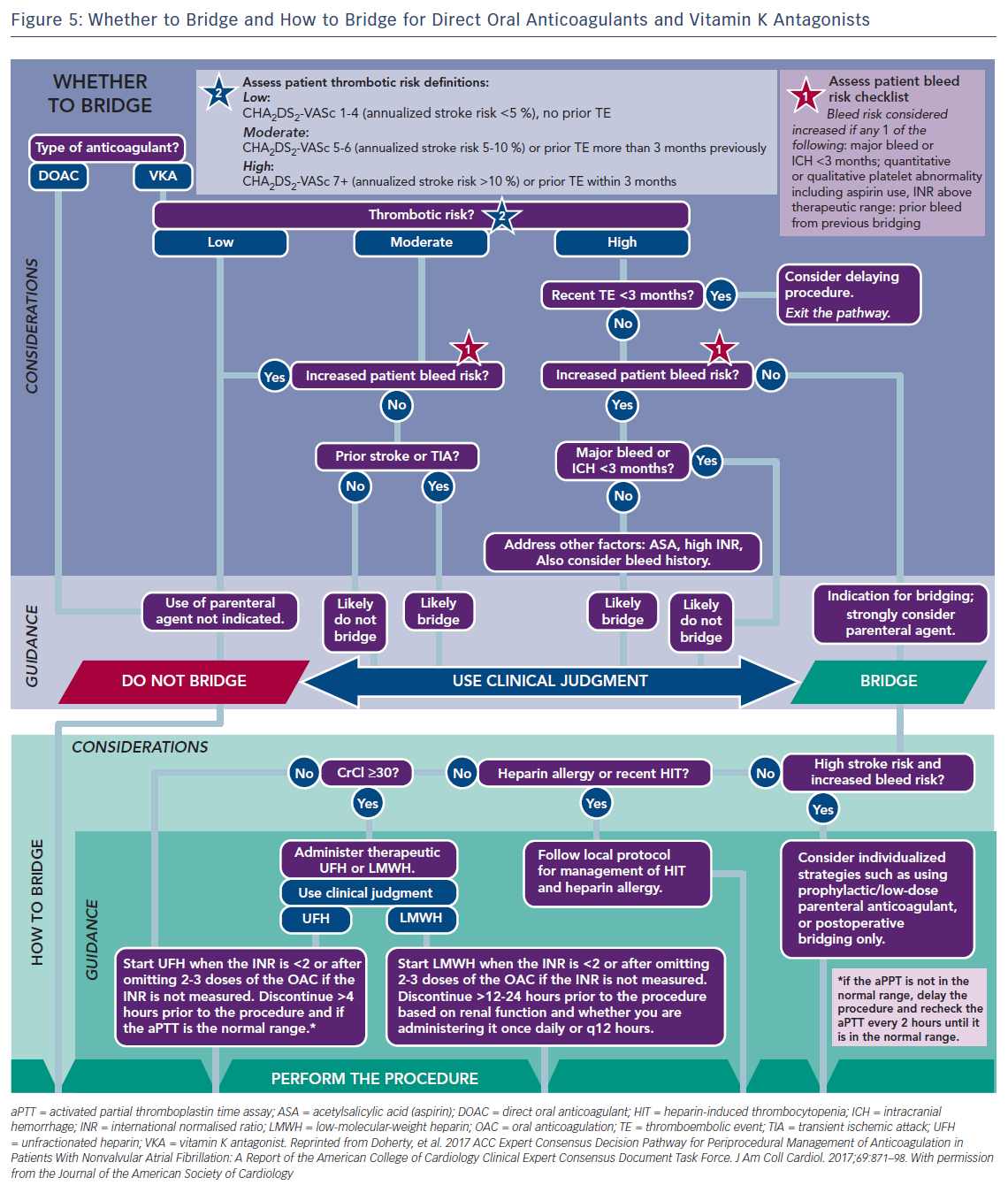
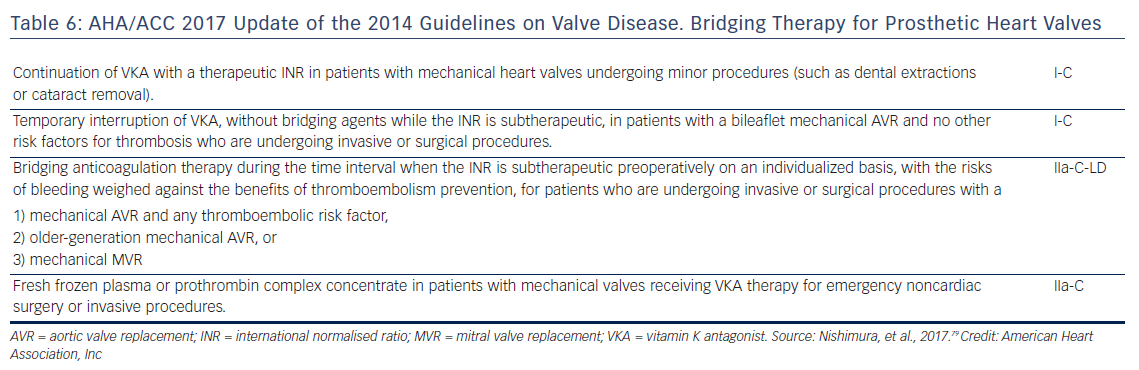
Usually major surgical procedures require an INR of at least <1.5. Warfarin has a half-life of 36–42 h and should be stopped for 3–4 days before surgery when the INR is <2 and 5 days when it is >2 (Figure 4).63 In urgent cases oral or IV vitamin K (1–2 mg) may be considered. In need of urgent reversal, prothrombin plasma concentrate may also be added, and is preferable to fresh frozen plasma.64 Bridging to UFH or LMWH that is discontinued ≥12 h before and restarted 24 h after the operation, has been recommended only in patients with certain mechanical heart valves and high risk of thrombosis (Figure 5 and Table 6). In a recent meta-analysis, however, heparin bridging for invasive procedures and surgery in patients receiving vitamin K antagonists for AF, prosthetic heart valves, or VTE conferred a greater than five-fold increased risk for bleeding, whereas the risk of thromboembolic events was not significantly different between bridged and non-bridged patients.65 The use of therapeutic dose LMWH was associated with an increased risk of bleeding compared with prophylactic or intermediate dose.65 Thus, bridging is not required, especially in patients at low risk of thrombosis.66–68 In the continued-warfarin group, the INR in the day of surgery should be ≤3, except for patients with one or more mechanical valves, for whom an INR ≤3.5 or less is permitted (Bridge or Continue Coumadin for Device Surgery Randomised Controlled Trial [BRUISECONTROL] trial).67 In patients with AF, normal renal function and platelet count platelet count >100 × 109/L, even major surgery can be safely accomplished with warfarin cessation without bridging when the INR is <1.8 (Effectiveness of Bridging Anticoagulation for Surgery [BRIDGE] trial). Warfarin is then resumed the evening after the procedure.68 There are limited data on safety of cardiac surgery or other major surgery with a very high risk of thromboembolism and bleeding, in patients who are on warfarin. Currently, these patients are bridged with heparin prior to surgery. In the need of emergent coronary artery bypass grafting (CABG), fresh frozen plasma and vitamin K may be used to reduce the risk of bleeding.
Non-vitamin K Anticoagulants
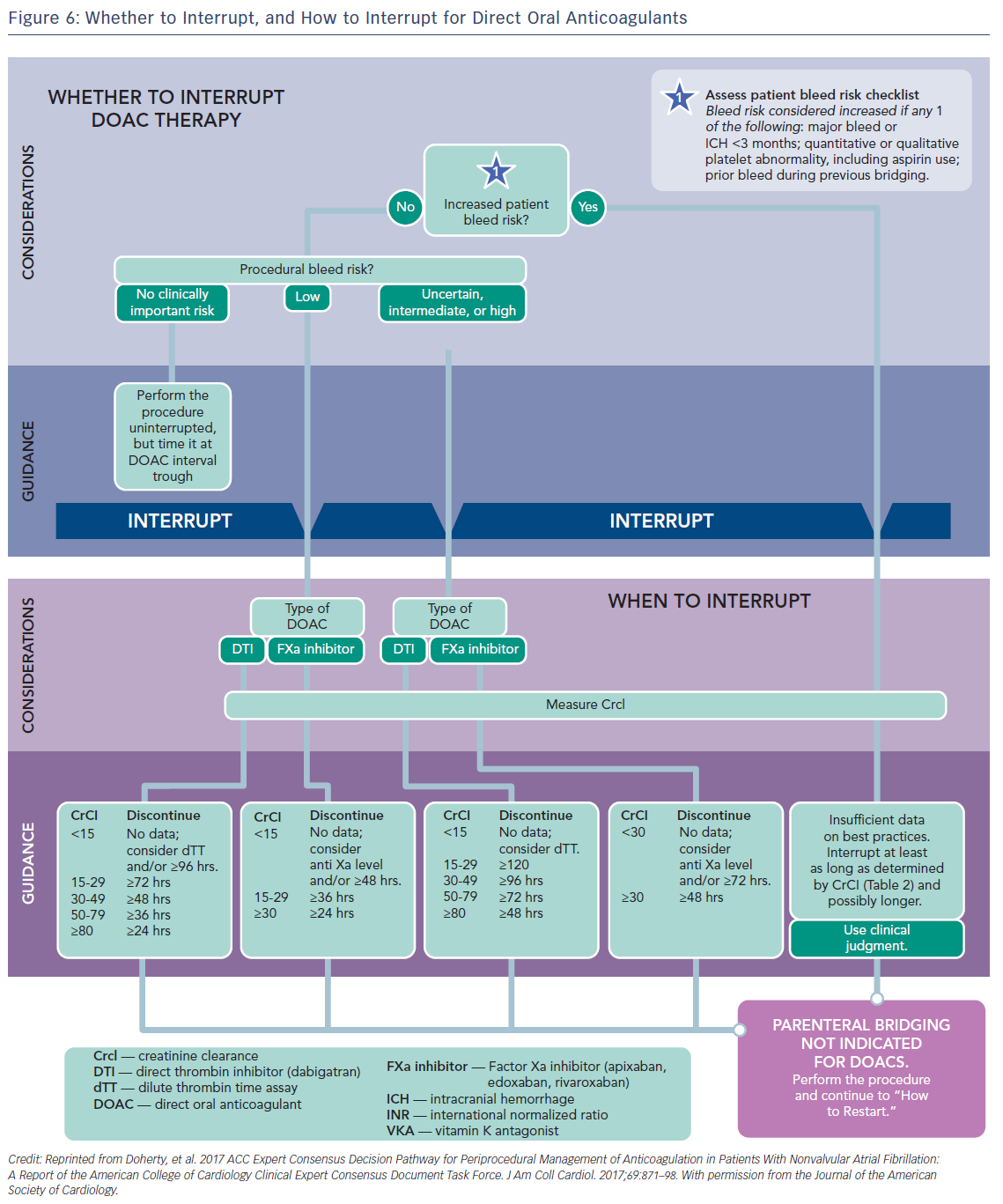
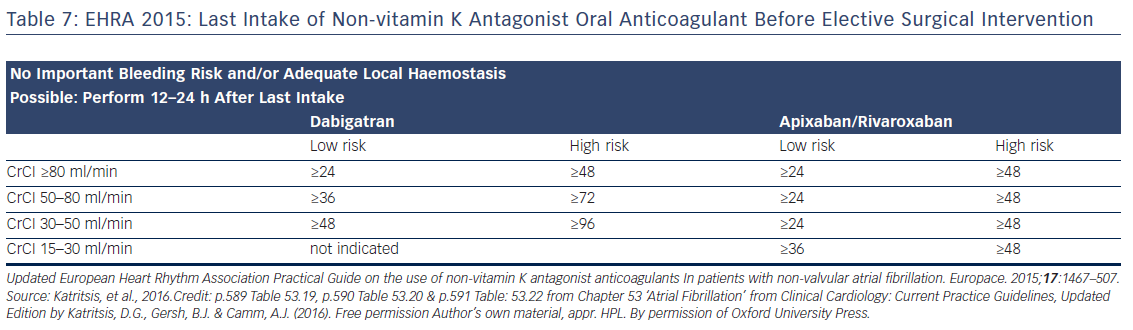
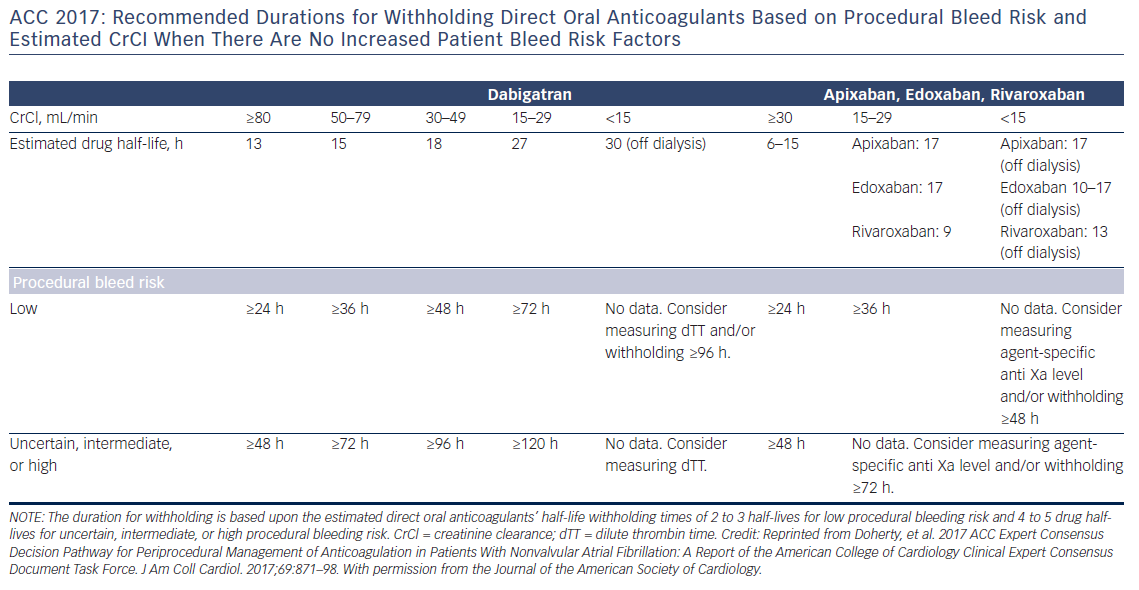
Preoperative interruption of DOAC depends on the risk of bleeding and renal function (Table 7 and Figure 6)52,63,69 In low-risk patients with normal renal function 24–48 h interruption of dabigatran and 24 h interruption of Xa inhibitors is sufficient. In patients at high bleeding risk dabigatran is interrupted 120 h (CrCl <30 ml/min) to 48 h (CrCl >80 ml/min), and Xa inhibitors 72 h (CrCl <30 ml/min) to 48 (CrCl >30 ml/min).63 There has been recent evidence that continuation or short-interruption of DOAC are safe strategies for most invasive procedures.70 Bridging with heparin is, on most occasions, not necessary and may increase the risk of bleeding.70 In an analysis of data from the Randomised Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial, interruption of dabigatran (2 days) or warfarin (5 days) for allowance of surgery was not associated by a significant occurrence of stroke and systemic embolism although heparin bridging was used in <80 % of patients on dabigatran, and major bleeding was not different in the two treatment groups.71 However, discontinuation of rivaroxaban in the ROCKET AF trial for at least 3 days was associated with a higher incidence of stroke compared to discontinuation of warfarin.72 Thus, in patients with a CHADS2DS2VASC score >4, i.e. >5 % annual risk of stroke, bridging therapy with LMWH should be considered. Procedures with low haemorrhagic risk (dental extraction, skin biopsy, cataract surgery) can be safely performed without interruption of NOACs, especially if carried out 12 h after last dosing. For pacemaker and ICD implantation a 24-h discontinuation with re-initiation 48 h after implantation (24 h in patients with a CHADS2DS2VASC score >4) is recommended.73,52
If urgent surgery or intervention is required, the risk of bleeding must be weighed against the clinical need for the procedure. Dilute TT for dabigatran and anti-Xa assays for rivaroxaban and apixaban are used to assess anticoagulant activity. aPTT and PT may also be used as rough estimates of the anticoagulant activity of dabigatran and rivaroxaban, respectively.28 Sensitive PT may be used as a rough estimate of the antocoagulation intensity of all FXa inhibitors. Specific coagulation test (dTT for dabigatran; chromogenic assays for FXa inhibitors) can also be considered, but no clinical experience exists.52 Surgery or intervention should be deferred, if possible, until at least 12 h and ideally 24 h after the last dose. Non-specific antihaemorrhagic agents, such as recombinant human activated factor VIIa or prothrombin complex concentrates should not be given for prophylactic reversal due to their uncertain benefit-risk.74 Re-initiation of these agents should be delayed for 24–48 h and once complete haemostasis is assured, since within 1–2 h of re-initiation the patient will be anticoagulated. For procedures with immediate and complete haemostasis NOACs can be resumed 6–8 h after the intervention.52
Clinical Perspective
- Patients on anticoagulation for atrial fibrillation are still at risk of ischaemic stroke, and may also develop haemorrhagic complications.
- Prompt diagnosis and therapy is necessary for these conditions.
- Specific antidotes are now available for the new, direct oral anticoagulants.