Deep brain stimulation (DBS) is an expanding neurosurgical treatment for refractory neurological conditions such as Parkinson’s disease and essential tremor, with over 120,000 devices implanted worldwide.1 The rate of cardiac implantable electronic device (CIED) implantation is rising annually, with 739 pacemaker implants per million and 141 ICD implants per million in western Europe in 2015.2 There are no data in the literature on the incidence of cardiac device implantation in patients with pre-existing DBS or vice versa. However, in an ageing population, the need for cardiac device implantation in patients receiving DBS is likely to become increasingly frequent. There is a theoretical risk of interference between these two systems as well as practical issues that must be taken into consideration during the implantation procedure.
In this article, we will discuss the indications and physiology around DBS and review the evidence in the literature for CIED implantation in patients receiving DBS. We will then summarise the potential interactions and practical considerations for patients with both systems, with advice for cardiologists in managing such patients.
Deep Brain Stimulation
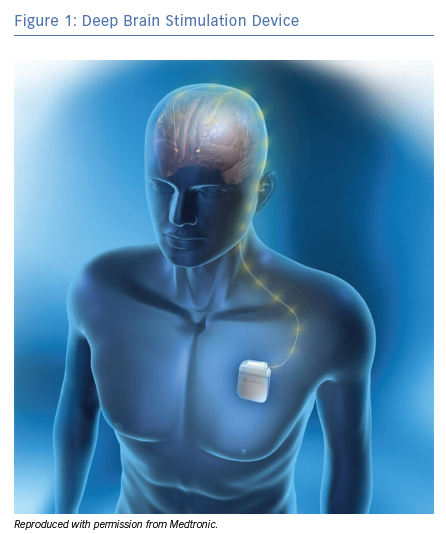
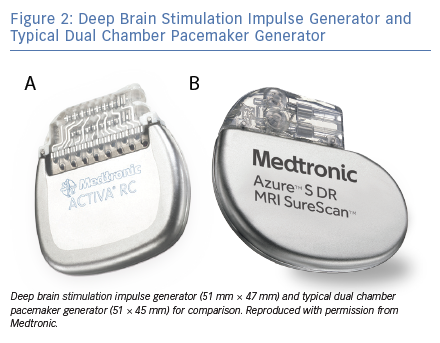
DBS is an intervention whereby electrodes are implanted through a neurosurgical procedure into stereotactically mapped brain regions, which are selected based on the indication, and connected by wires to an implanted pulse generator (IPG; Figure 1 and Figure 2). The IPG is usually situated in the left subclavicular region. Bilateral brain electrodes can be connected to either separate bilateral IPGs or to a single IPG.
Before the long-term electrode is implanted, physiological verification is carried out by passing a microelectrode into the anatomical target to record neuronal activity, and short intervals (0.5–1 second) of high-frequency test stimulation (100–300 Hz) are delivered to the awake patient intraoperatively to observe both beneficial and adverse effects. The procedure is therefore initially carried out under general anaesthesia, with sedation interrupted for microelectrode recording. Several weeks following the procedure, the IPG is programmed by a neurologist to determine the optimal settings, with voltage, amplitude and pulse width usually manipulated.3,4
DBS is being used for increasingly wide applications. It is most commonly used for movement disorders such as Parkinson’s disease, essential tremor and dystonia when medical treatment is ineffective or causes intolerable adverse effects. It has also been used experimentally for treating psychiatric disorders (such as refractory depression and obsessive–compulsive disorder), obesity and chronic pain states.5 It is best established for late-stage Parkinson’s disease in patients who are levodopa sensitive, where the subthalamic nucleus or internal segment of the globus pallidus are targeted unilaterally or bilaterally. The goal in these patients is to improve their motor function and reduce their levodopa-effective dose.
The precise mechanism of DBS is debated. It is theorised that in Parkinson’s disease, DBS at a high frequency can override the pathological low-frequency, beta-wave, oscillatory activity (11–30 Hz) and start a higher rate of neuronal activity (>60 Hz) in the dopaminergic pathways, which can treat symptoms such as bradykinesia and rigidity. Conversely, high-frequency stimulation may hold the low-frequency oscillations underlying tremor in a refractory state, thus helping to relieve this symptom.6 Hence, traditionally, a high frequency (>100 Hz) has been used, although this may be different in select patients.7
Literature Review
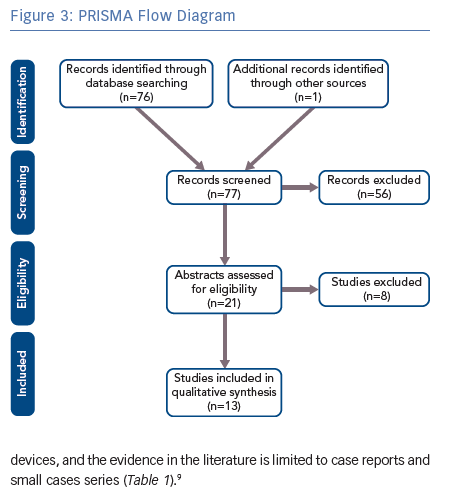
A systemic review of the literature was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.8 We performed a comprehensive search of major biomedical databases (including PubMed, EMBASE and AMED) using variations of the following terms: ‘deep brain stimulation cardiac pacemaker’; ‘neurostimulator cardiac pacemaker’; ‘deep brain stimulation implantable cardioverter-defibrillator’; ‘neurostimulator implantable cardioverter-defibrillator’; and ‘deep brain stimulation cardiac defibrillator’. The final search was conducted on 10 September 2018. The reference lists from the included articles were also searched.
We used a focused search strategy to include all studies that referred to DBS and cardiac pacemakers or ICDs. Prior to the search, inclusion and exclusion criteria were determined. Articles were included if they were primary studies and referred to the use of DBS and pacemakers or ICDs. Articles were excluded if they were individual views, such as commentaries or letters, or if they were secondary research, such as literature reviews. Electronic abstracts were screened using the inclusion and exclusion criteria.
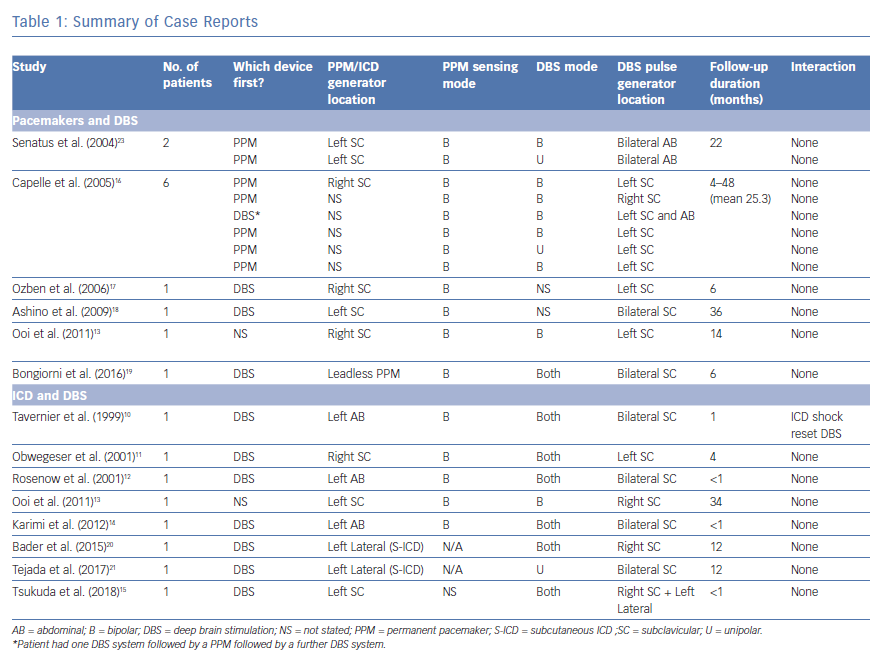
After applying the inclusion and exclusion criteria, we included six articles for pacemaker and DBS and eight articles for ICD and DBS. The methodology is summarised in Figure 3. The cases described in these articles are summarised in Table 1.
Current Evidence
While device manufactures have provided some guidance, there are no specific guidelines for the implantation of CIEDs in patients with DBS devices, and the evidence in the literature is limited to case reports and small cases series (Table 1).9
The first description of a CIED in a patient with DBS was by Tavernier et al. in 1999.10 The patient had bilateral DBS with IPGs in both left and right pectoral regions. An ICD was implanted in the left abdominal position for secondary prevention after a cardiac arrest. While the DBS devices did not have an impact on the ICD function, a 34 J shock from the ICD was found to completely reset both DBS devices, resulting in them reverting to the ‘off’ position.
Five further studies reported successful implantation of ICDs in patients with DBS; however, in these cases, there was no reported interaction between the devices, with DBS function continuing as normal after defibrillator threshold testing (DFT) or ICD shocks.11–15 Cases of cardiac pacemaker implantation in patients with DBS have, similarly, found no adverse interactions between the two systems.13,16–18
More recently, novel devices have been employed in patients with bilateral DBS pulse generators who require ICD or pacemaker implantation. Borgioni et al. described the implantation of a leadless pacemaker in a patient with bilateral BDS without any reported interaction.19 Bader and Weinstock, and Tejada et al. demonstrated the successful implantation of subcutaneous ICD (S-ICD) systems in patients with DBS, again with no interference between the devices.20,21
Potential Interactions
Several practical and theoretical interactions should be taken into consideration for patients with both DBS and a cardiac device. The most concerning of these is the potential for one of the devices to impact on the activity of the other.
One study reported the complete reset of bilateral DBS devices after a shock from an implanted ICD; however, there have been no other reports of similar interactions.10 While brief interruption of DBS is unlikely to cause significant morbidity or mortality, the inhibition of a cardiac pacemaker or ICD could result in syncope or sudden cardiac death. ECG artefacts have been reported in patients with DBS and detection of such activity by a cardiac device could potentially inhibit pacing or trigger an inappropriate ICD shock.22
There have been no reports of any inappropriate sensing by a CIED in patients with DBS. Nevertheless, several precautions can be taken to minimise the risk of any interference. Medtronic recommends that both the DBS and cardiac device sensing should be programmed to a bipolar configuration.9 Ensuring the CIED is in bipolar mode for sensing significantly reduces the size of the electrical circuit, thus decreasing the potential for it to detect the DBS signal.
Capelle et al. demonstrated that changing the atrial sensing configuration of a cardiac pacemaker from unipolar to bipolar significantly reduced the intracardiac ECG artefact from a unipolar DBS system, though neither configurations resulted in inappropriate atrial sensing.16 The vast majority of modern CIEDs are programmable to bipolar sensing, but this is an important consideration for patients with older devices. There are no reported cases in the literature where a patient with a DBS device has a cardiac device using unipolar sensing configuration. S-ICDs have much larger bipoles for sensing compared to transvenous systems; however, two cases demonstrated no evidence of inappropriate sensing in patients with DBS.20,21
DBS systems can also be programmed to either unipolar or bipolar mode. When in bipolar mode, the circuit is small and limited to the electrode implanted in the brain. In unipolar mode, the circuit is much larger, running from the brain electrode down to the IPG in the chest. It is generally recommended that both the cardiac device and DBS device are set to bipolar mode, and it has been shown that doing so eliminates any interference on both the surface and intra-cardiac ECG.13,16,23 The setting of the DBS, however, depends on the requirements of the patient, and can change the efficacy of the treatment, with some patients requiring unipolar configuration for optimal symptom control. Unipolar configuration of a DBS system has been reported along with a CIED without any significant interference.10–12,15,16,19–21,23
A further precaution to take is to ensure there is an adequate distance between the generators of DBS and cardiac devices. Medtronic recommends that the DBS IPG and CIED generator are implanted on contralateral sides at a distance of at least 20 cm.9 This recommendation is seen elsewhere in the literature.11,13,16,17,24 While there are reports of generators being implanted in close proximity without any interaction, ensuring a maximum distance between the two minimises the potential for interference in electrical circuits.18 One study reported a possible interaction between a DBS IPG and a cardiac pacemaker that were close to each other.24 This patient had a bilateral DBS system with IPGs in both subclavicular regions as well as a left-sided cardiac pacemaker. They presented with neurological symptoms of imbalance, tingling and weakness, which resolved when one IPG was moved from the left subclavicular to an abdominal location. While the authors suggest this had been an interaction between the DBS and cardiac pacemaker, there was no objective evidence of device malfunction.
After implantation of a CIED in a patient with DBS (or vice versa), the programmed parameters of each device should be checked. It is prudent to programme the devices for ‘worse case scenario’ events. This involves trialling both unipolar and bipolar settings for the DBS, and programming the DBS to maximum tolerated settings and the cardiac device to the maximum sensitivity.13,23 Medtronic recommends that the DBS is set to a minimum frequency of 60 Hz.9 For ICD implantation, defibrillator threshold testing can be used to detect any impact of ICD shocks on DBS settings or function.
Practical Considerations
During the actual implantation of a cardiac pacemaker or ICD in a patient with DBS, there are several factors to take into consideration.
The DBS system incorporates unilateral or bilateral electrodes implanted into the brain with wires running subcutaneously down to an IPG, which is usually located in the subclavicular region. CIEDs should be implanted on the contralateral side to the IPG, not only to avoid potential interaction as discussed, but also because there is unlikely to be sufficient physical space on the ipsilateral side for two generators.
Furthermore, this minimises the risk of damaging the DBS system during implantation or future box changes. Ensuring adequate physical separation between the generators also reduces the chance of inadvertent disturbance of the incorrect system when using magnet or electronic programming devices.13 Some patients have bilateral IPGs occupying both subclavicular spaces, which creates an additional challenge for cardiac device implantation. In such cases, options include: relocating one of the IPGs; implanting the CIED generator in an abdominal position; or using novel devices such as leadless pacemakers or S-ICDs.10,12,14,15,19–21
Another issue during cardiac pacemaker or ICD implantation is the use of electrical diathermy. This is a common tool used for both dissection and haemostasis. Diathermy can potentially interfere with the activity of the DBS, though brief interruption of the system is unlikely to have much of a clinical effect. Of more concern is that the energy from diathermy can theoretically be transferred via the DBS system to the brain and cause damage at the site of the implanted electrode.25,26 There has been a case report of severe central nervous system damage secondary to an interaction between diathermy and DBS.27 It is therefore wise to avoid diathermy in patients with DBS systems. If diathermy is absolutely required, options include turning off the DBS device before the procedure or ensuring bipolar diathermy is used.
Conclusion
While the reports of cardiac pacemaker or ICD implantation in patients with DBS are limited, the evidence suggests that both systems can be implanted without significant interaction, provided that certain precautions are taken.
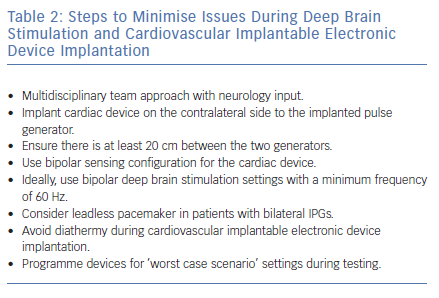
Patients receiving DBS who require CIED implantation should be managed in a multidisciplinary setting with input from neurologists to optimise DBS settings prior to the procedure. Several practical steps can be taken by the cardiologist to minimise the risk of interference (Table 2).
Since the published outcome data in patients with cardiac devices and DBS is limited to case reports, it is possible that significant interactions are under-reported. A large, prospective, observational study is needed in this patient population to more clearly assess safety and accurately determine the risk of complications and interaction between cardiac devices and DBS.
Clinical Perspective
- Cardiac devices can be safely implanted in patients receiving deep brain stimulation (DBS) provided that certain precautions are taken.
- Patients should be managed with a multidisciplinary approach including neurology input.
- Cardiac device generators and DBS implantable pulse generators should be implanted on contralateral sides with >20 cm distance between them.
- Both the cardiac and DBS systems should be in bipolar configuration.