The number of catheter ablations for atrial fibrillation (AF) treatment has gradually increased over the last 15 years since the first report on the importance of pulmonary vein (PV) foci for triggering AF.1 Catheter ablation for AF is a complex procedure with multiple steps, such as transseptal puncture, mapping of the left atrium and PVs and extensive linear ablation around PV ostia. Not surprisingly, this procedure has been associated with higher radiation doses than conventional ablation procedures.2–4 Over the last two decades, catheter ablation has evolved from an almost experimental and uncertain procedure to a routine and well-established practice worldwide. However, literature data reveal significant differences among individual centres regarding the use of fluoroscopy time (FT) and radiation dosage per procedure. From the patient’s point of view, the average 1 hour of FT during AF ablation is estimated to increase the risk of a fatal cancer by up to 0.1 %.4 In addition, patients with AF may require repeat procedures that contribute to a high amount of total lifetime radiation exposure.5,6 From the physician’s point of view, the radiation exposure dose may represent only 1–2 % of the patient’s dose. Nevertheless, a high volume of ablations per operator is associated with a higher probability of receiving a substantial radiation dose.7
Radiation exposure has been associated with malignancies, cataracts, thyroid dysfunction and other diseases.8–19 Hence, every electrophysiologist should know how to lower radiation exposure. Here we review the basics of X-ray and discuss the health hazards induced by radiation exposure. We also focus on the current strategies being used to decrease X-ray dosage, emphasising the role of nonfluoroscopic catheter-guiding technologies.
X-ray Physics and the Principle of Imaging
The physics of X-ray imaging is based on the interaction of X-rays with matter. X-rays are composed of both electromagnetic waves and particles (photons) that are powerful enough to penetrate deeply into and through matter. In the human body, this interaction results in a shadow image, where X-rays are emitted from a point source and absorbed differently in various tissues. This absorption effect is called the radiation dose, which is inherent to X-ray imaging. The radiation dose is defined as the energy released in reference matter. For the entrance dose before the skin, the reference material is air and, therefore, measurement of radiation energy absorbed in air volume is called air kerma (kinetic energy released per unit of mass), measured in J/kg or Gray (Gy). Although energy released in matter is small, it has biological effects. Sievert (Sv) is the unit used to evaluate the impact of biological ionising radiation on biological tissues. In the case of X-rays and biological tissue, 1 Gy is equal to 1 Sv.20
X-rays interact with human tissues in different ways. Some X-rays deviate with or without changes in energy, a process known as scatter. Other X-rays penetrate the tissue or are completely absorbed. Scatter has two unfavourable consequences. First, it blurs the shadow image originating from one point source. Second, it exits the body of the patient in different directions and delivers a radiation dose to other individuals in the near vicinity.21
Equipment that produces X-ray images consists of an X-ray tube, an image detector with an anti-scatter device on the other side and a table between them supporting the patient. An X-ray tube emits X-ray photons with a continuum of different energies. In principle, the highest energy (in keV) corresponds to the highest voltage applied to the X-ray tube (kVp).
Radiation Exposure Induces Health Hazards
Biological effects of radiation exposure can be viewed as a function of time. Part of the X-ray beam energy is absorbed in the tissue within milliseconds. This induces the release of energy inside tissues, causing biological effects within seconds. These effects comprise two mechanisms. One is direct cellular damage, represented by DNA breakage, especially in dividing or immature cells. The other is indirect, due to hydrolysis and the formation of free radicals, ultimately leading to cell apoptosis. The DNA repair system can correct some of these effects, although high or repeated exposure leads to irreparable changes such as DNA double-strand breaks.
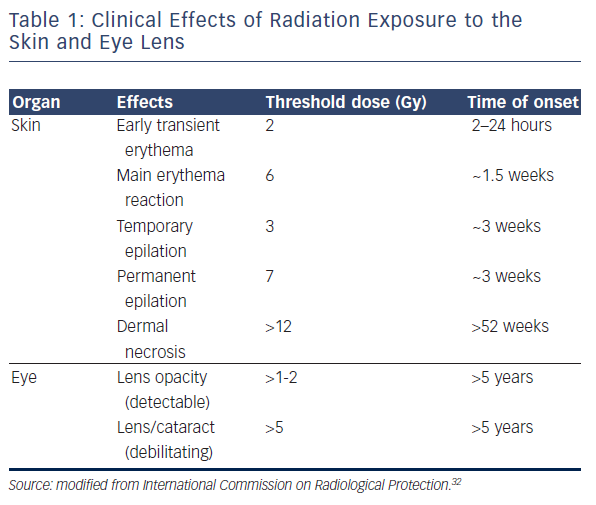
Two types of biological effect can be differentiated with variable clinical outcomes. Deterministic effects occur once the radiation dose exceeds a specific threshold, and their severity becomes linear to the dose.22 Significantly, although there is a threshold, the actual value may change according to previous exposure or the patient’s health condition (including use of prescribed drugs) and this should be taken into consideration.23–26 The skin and eyes are the best known organs that are vulnerable to X-ray with deterministic effects (erythema, skin burn, hair loss, necrosis and cataract), with a suggested threshold of 2 Gy and 500 μGy, respectively.22,27–32 The International Commission on Radiological Protection has outlined the correlation between radiation dosage and its effect on each organ (see Table 1).32,33
Regarding the onset of clinical manifestations, effects can be prompt (<2 weeks after the procedure), early (2–8 weeks), mid-term (6–52 weeks) and long-term (>52 weeks). Obviously, there is significant individual variability in manifestations and their timing.
In one study, the lifetime risk for fatal malignancies after 1 hour of fluoroscopy for an ablation procedure was estimated at 0.07 % and 0.1 % for male and female patients, respectively.4 Organs at highest risk were the lungs, stomach, active bone marrow and, in female patients, breast tissue. As this risk calculation was performed at a setting of 7.5 frames per second, no collimation use and the left anterior oblique (LAO) and right anterior oblique (RAO) in their preference, using the techniques mentioned later may reduce the risk to an extremely low level.
Stochastic effects are not associated with a particular threshold and cannot be predicted. Therefore, these effects are probabilistic in nature and their occurrence is not threshold dependent. Their onset increases proportionally according to the intensity of exposure, but not in terms of severity. Malignancies and genetic diseases are prone to occur, but the severity of the diseases cannot be determined by dosage.22 A recent report showed a higher incidence of glioblastoma multiforme at the left hemisphere among interventionists.34 As this study was only a voluntary reported case series, the true incidence of the tumour among physicians is unknown. Nevertheless, it is of interest that glioblastoma is related to radiation exposure and that the incidence was higher in the hemisphere facing the X-ray tube. Another survey reported that breast cancer that is also sensitive to radiation exposure was more frequent in the left side among female cardiologists.28
Dose Metrics and Measurements
Several parameters are used to express and monitor patient dosage, of which the most important are FT, dose area product (DAP) and cumulative air kerma at the reference point.
FT is displayed on all interventional fluoroscopy systems. FT accounts for all of the time spent using fluoroscopy and thus can be viewed as a quality assurance measure for the physician and a definite procedure. However, it is well known that FT poorly correlates with other dose indicators.35,36 In addition, it can be calculated differently depending on the manufacturer (either total time with pedal pressed or just a sum of the X-ray pulses).
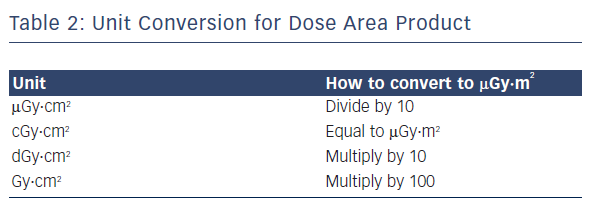
DAP, or the kerma area product, is defined as the product of the air kerma (the energy extracted from an X-ray beam per unit mass of air) and the area of the cross-section of the X-ray beam. It is a surrogate measurement for the entire amount of energy delivered to the patient by the beam, measured in μGy·m2.37Table 2 shows conversion rates for commonly used units.
How to Decrease Radiation Exposure
There is a difference in the origin of radiation exposure between patients and physicians. For patients, the primary beam is the source of radiation exposure. On the contrary, as the X-ray passes forward straight from the X-ray tube to the detector, the main source of physicians’ exposure is the X-ray emitted from the patient via photoelectric effect and Compton scattering. The latter Compton scattering gives the largest radiation dose to the physician and is a phenomenon of X-rays interacting with atoms, thus scattering radiation in various angles.
Reduction of X-ray Dose and Time
Optimising the Fluoroscopy System for Electrophysiology Procedures
Many physicians may believe that there is no need to modify system settings. As fluoroscopy systems are manufactured for various percutaneous procedures, they can be adjusted, particularly for electrophysiology (EP) procedures. One big difference between the settings used by electrophysiologists and other interventionists is that electrophysiologists do not need precise images during the procedure. They can navigate within the heart to a great extent using electrograms and/or non-fluoroscopy mapping or imaging systems. One major difference is the frame rate for fluoroscopy, which is usually set to 15 frames per second in the angioplasty laboratory compared with 2 frames per second in our laboratory.
Soft X-rays are usually absorbed or scattered by photoelectric effect and have the least impact on image quality. By adding a copper or aluminium filter, we can omit these X-rays resulting in a 30–50 % reduction of the radiation dose.38,39 Many systems automatically add or remove the filter according to image brightness by automatic dose reduction control (ADRC). New systems are obligatorily equipped with these filters. However, in the case of an old fluoroscopy system it is worth asking the provider for filter settings.
A secondary radiation grid placed in front of the detector is essential for sharpening the image by omitting the scattered X-ray. The direct effect of solely removing this grid in the EP field was studied in a phantom model and resulted in a reduction of roughly 50 % of the radiation dose, irrespective of the fluoroscopy angle.40 This study also analysed the effect in clinical cases, where image quality seemed to be acceptable as only 5 out of 417 cases required the grid to be replaced during the procedure. Especially in obese patients in whom X-ray is more scattered, DAP with and without the grid increases the radiation dose twofold.41
There are a few more changeable settings from the software side. Tube voltage, pulse duration and radiation limitation by ADRC can be changed in some systems. Although changing these settings invariably results in lower image quality, electrophysiologists do not need such high quality, and lower resolution images are usually acceptable once they get used to the image.42,43 The number of factors that can be modified in the individual system varies and should be clarified with the vendor.
Compliance With General Principles of Radiation Protection
Collimation reduces image area and decreases the DAP linearly according to the narrowed field. An effort to maximise collimation can decrease the radiation dose by up to 12 %.44 However, many electrophysiologists do not use collimation at all during procedures. Asymmetric collimation can achieve another 60–80 % reduction compared with normal collimation, although it has not yet been commercialised.45 In contrast, magnification seems to show the same area as collimation, but increases the radiation dose by ADRC and increases DAP. Magnification should generally be avoided in EP procedures.
Projection angle is another important parameter as angling to low LAO (50–60°) compared with shallow RAO (30°) positions results in a twofold increase in X-ray dose received by the patient.4,46–48 This difference is due to the fact that in LAO, the X-ray passes through the liver, vertebral column and mediastinum, which require a higher dose of X-rays to permeate, whereas in RAO the X-ray mainly passes through the lung containing air allowing the X-ray to permeate easily. To the physician, LAO causes more scattered radiation as the X-ray tube approximates the physician increasing the exposure dose up to sixfold compared with RAO.46 Based on our experience, we believe that the use of lowoblique projections during catheter ablation of AF is rarely required.
Both raising the patient’s table by 10 cm and positioning the detector 10 cm nearer to the patient result in approximately 15 % reduction of the radiation dose.11,49
The frame rate may also be changed. As radiation exposure increases per frame in a linear way, it should be set as low as acceptable. The most ‘aggressive’ setting uses an ECG-triggered pulse, which can potentially decrease the frame rate below 1 per second, depending on the heart rate. In our experience, 2 frames per second is a reasonable compromise for use during EP procedures.
Reduction of X-ray Usage Time
Even if attempts are made to decrease the X-ray dose/time, it can be counterbalanced by longer fluoroscopy usage. To avoid this, the use of unnecessary fluoroscopy should be avoided.
There are other technologies that can reduce fluoroscopy time and the radiation dose during complex catheter ablations, such as ablation for AF. Some employ a 3D electroanatomic mapping system (e.g. the CARTO® system [Biosense Webster] using magnetic fields or the EnSite™ NavX™ system [St. Jude Medical] using transthoracic currents).50,51 These systems can project the catheter tip in real-time on a virtual 3D image, limiting the need for fluoroscopy.52–55 In the CARTO system, when real-time catheter positioning is achieved by updating CARTO-XP® to the CARTO® 3 version, it results in a significant reduction of FT, which may contribute to a reduction of the radiation dose.56,57 One limitation of 3D mapping systems is that they only provide virtual images of the heart, which may differ from real-time anatomy.58,59 To compensate for this and to visualise catheter shaft or sheath positions, fluoroscopy still needs to be used intermittently.
Another useful strategy for reducing radiation during complex catheter ablation procedures is intracardiac echocardiography (ICE).60–62 ICE provides a real-time image of the heart, which is different from 3D mapping systems.63 CARTO 3 and ICE-derived CARTOSOUND® (Biosense Webster) can be combined to compensate for the limitation of the solo 3D mapping system by rapidly updating the anatomical map during the procedure.64 There are preliminary studies that have combined 3D mapping systems and ICE, leading to the use of zero fluoroscopy ablation procedures.65,66 The largest limitation of ICE is the high cost of the probe, which must be discussed in terms of the balance between the above merits in each facility.
Contact force-sensing catheters also have the advantage of reducing radiation time. When they are integrated with the 3D mapping system, electrophysiologists can control catheter-tissue contact without confirming by movement of the catheter tip on fluoroscopy.67,68
Recent advances in technology, such as CARTOUNIVU™ (Biosense Webster) and MediGuide™ (St. Jude Medical), have increased the potential for decreasing radiation exposure.69–71
Changing Settings in Real-world Practice
One recent report used a detector entrance dose setting of 8 nGy for fluoroscopy and compared it with 23 nGy when ablating complex left atrial arrhythmias.72 They achieved a great reduction in DAP from 878 μGy·m2 to 200 μGy·m2 in their study, which is lower than previous reports ranging from 789 to11,199 μGy·m2.69–73
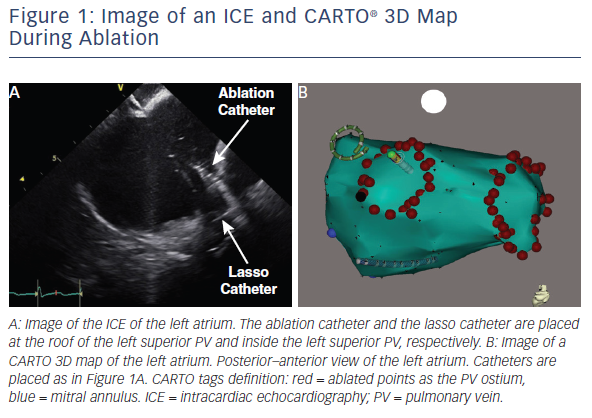
In our institution, we use 23 nGy for the detector entrance dose. We comply with collimation and avoid oblique views as much as possible in combination with 3D mapping systems and ICE (see Figure 1). Due to these efforts, our average DAP for catheter ablation of AF was 252 μGy·m2 among 113 patients.74
One can argue that these adjustments may reduce radiation exposure, but can also inversely increase complications due to image deterioration. However, this has not been our experience while using ICE imaging. In a large series of cases of catheter ablation for AF from our centre, the rate of potentially life-threatening complications was <1 %.75 However, we recommend making step-by-step changes and getting accustomed to each setting before changing all of the settings at once.
Remote Navigation System for Preventing Radiation Exposure to Physicians
Remote navigation systems can be used to reduce radiation exposure, mainly for the physician. Two types of remote navigation systems used to manipulate catheters with a magnetic field or a steerable sheath are currently available. Clinical outcomes are comparable to manual manipulation, even though the procedure time is longer using remote navigation systems.76 There are still limited data in this field, but FT and DAP are consistently lower using remote systems.77–85 Intuitive navigation implemented in remote navigation suites appears to be one possible explanation. Remote navigation seems to be easier than manual manipulation as there is less of a need for fluoroscopy. This results in the reduction of DAP for the patient. As the physician remains remote from the X-ray tube during ablation, radiation exposure is avoided.86
Magnetic Resonance Imaging-guided-based Electrophysiology Intervention
Real-time magnetic resonance (MR)-based EP is another option for reducing radiation exposure, although the technique is still in the investigation phase.87,88 The speed of acquiring the image still requires more time compared with X-ray imaging. The most important concern for electrophysiologists may be the quality of intracardiac electrogram during MR usage. Besides these technical disadvantages that have to be improved, the largest advantage of this technique is not only the X-ray-free procedure, but also acquiring the intra-procedure ablation lesion formation. This technique may facilitate reaching a clear ablation endpoint as the ablation lesion can be directly visualised.
Personal Protective Equipment and Devices
Effective and proper use of personal protection is obligatory for all members of the EP laboratory to minimise radiation exposure. General protection, such as lead aprons, neck collars and shielding screens, are considered obligatory.89 To avoid orthopaedic complications associated with heavy aprons, the use of separated lead aprons or lightweight aprons are recommended with an equivalent effect. A thyroid collar is especially recommended when aged <40 years as the risk of thyroid cancer is high. Other small protectors, such as leaded glasses and leaded gloves, are less effective and efforts to lower radiation exposure need to be balanced against the discomfort of wearing them.90–92 Some centres use radiation-protection units, such as radioprotection cabins (Cathpax®, Lemer Pax), which protect from almost all scattered radiation without the need to wear lead aprons during the procedure.93 Another protector is a suspended lead apron mounted on the ceiling or on a floor unit (Zero Gravity™, Biotronik).94
As these protectors are designed to avoid Compton scattering, there is almost no effect on the patient as the body is the origin of the radiation.
Conclusion
Although the use of X-ray is still common during the majority of AF ablation procedures in clinical practice, it is important to realise that radiation can be harmful for both patients and physicians. By complying with the general principles of radiation protection and using new technologies, significant reduction of radiation exposure can be achieved. The principle of ‘As Low (radiation) As Reasonably Achievable (ALARA)’ should become the gold standard in any EP unit.
Clinical Perspective
- The number of atrial fibrillation ablations is increasing.
- The use of X-ray poses a relevant health risk, both to patients and physicians.
- By increasing the awareness of the physician and changing the system settings, radiation dosage can be decreased.