Atrial fibrillation (AF) is the most common clinical arrhythmia worldwide and is expected to increase in the coming decades.1,2 It currently affects up to 3 % of Western populations aged 20 years or older, and the number of affected individuals in the EU will increase from about 7 million to almost 13 million by 2030.3–5 This growing epidemic is not only caused by the natural ageing of the population, but also by the accumulation of chronic cardiovascular diseases and risk factors, and thus at least in part is caused by inadequate lifestyle.5–7
AF is a chronic condition and is independently associated with increased morbidity and mortality, including ischaemic stroke, dementia, cognitive dysfunction, heart failure (HF), MI and all-cause mortality.8–14 Stroke and HF can even be the first manifestation of AF. Although AF can be completely asymptomatic, about two-thirds of patients experience at least intermittent symptoms, which can be disabling and markedly impair health-related quality of life.15,16 AF-related symptoms and complications, as well as underlying cardiovascular diseases, lead to unplanned hospital admissions in a substantial number of patients every year.17,18 Therefore, it is not surprising that inpatient AF care accounts for more than two-thirds of the annual direct costs of AF and is the major cost driver.19–21
Contemporary AF Management
AF treatment has largely focused on the prevention of stroke and HF as well as symptom control, as reflected in previous European and current American guidelines for the management of AF.22–24 However, these guidelines make only narrow recommendations on upstream therapy in selected patient groups, e.g. treatment with angiotensin-converting enzyme (ACE) inhibitors or angiotensin II-receptor blockers (ARBs) in patients with HF or hypertension, or statin treatment in patients with postoperative AF. In contrast, contemporary AF management as outlined in the current European guidelines pursues an integrated approach with five domains that have to be individually addressed according to the needs of each patient.25 These five domains include: acute rhythm management in patients presenting with hemodynamic instability; detection and treatment of underlying predisposing conditions; stroke risk assessment and oral anticoagulation for stroke prevention; rate control; and rhythm control. The second domain now puts upstream therapy into a much broader perspective. The present review focuses on the detection and treatment of associated diseases and risk factors, i.e. the targeting of underlying conditions.26
Early Detection and Treatment of Underlying Conditions
AF is commonly considered a progressive disease – developing from a paroxysmal, self-terminating form through persistent to permanent AF – and is perpetuated by on-going electrical and structural remodelling of the atria.27–29 Some patients already have persistent AF at the time of first diagnosis.30 Registry data showed that patients with progression from paroxysmal to more sustained AF were more frequently admitted to hospital due to cardiovascular causes and had more strokes, but were also older and had a larger number of underlying comorbidities such as hypertension, HF, coronary artery disease (CAD) and previous stroke or transient ischaemic attack (TIA).31,32 A pooled analysis of the non-anticoagulated populations from the Atrial Fibrillation Clopidogrel Trial with Ibersartan for Prevention of Vascular Events (ACTIVE-A) and Apixaban Versus Acetylsalicyclic Acid to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment (AVERROES) trials found that patients with persistent or permanent AF at baseline had significantly higher stroke rates than those with paroxysmal AF. Findings from recent population-based studies and registries also demonstrated that at least 25–30 % of all patients with an ischaemic stroke and >80 % of those with cardioembolic ischaemic stroke also had AF, suggesting a strong association between these two entities.3,33–40 Another important finding in this context was that stroke was the first manifestation of previously unknown AF in >25 % of AF-related strokes.3,33–35,41 This association was even higher if prolonged noninvasive or invasive monitoring was performed following a stroke.42,43 Taken together, these findings call for earlier diagnosis and comprehensive treatment of AF to reduce stroke risk and improve outcomes.30
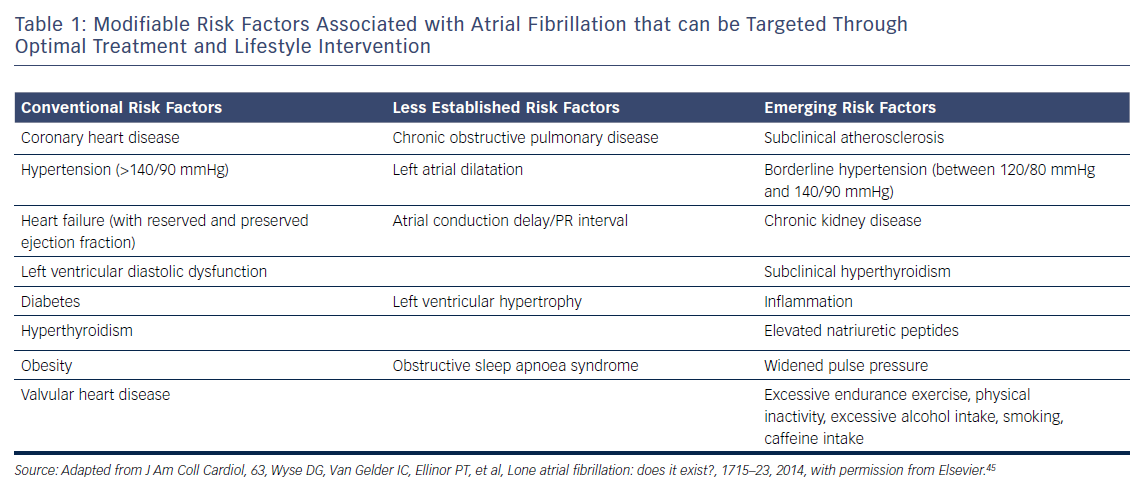
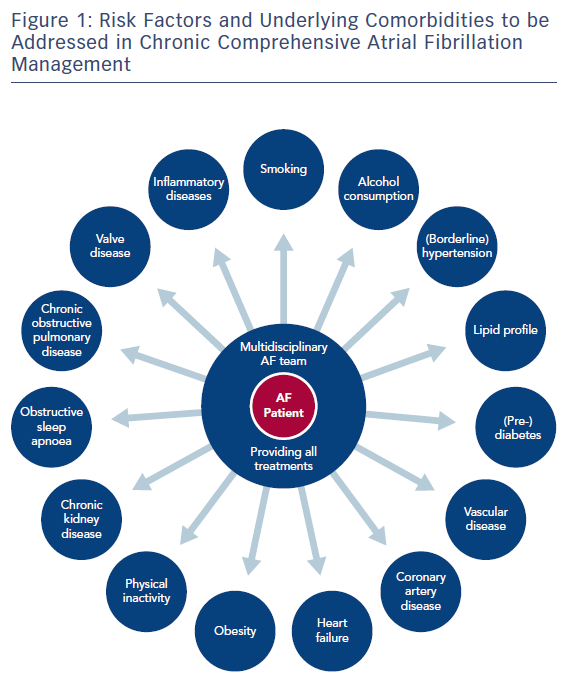
In recent years, a number of risk factors and conditions have been identified that are associated with the development and progression of AF.7,44–46 A few of these risk factors and predisposing conditions cannot be modified, such as advancing age, gender, ethnicity and genetic predisposition; however, most are modifiable or can at least be optimally treated (Table 1; Figure 1). Many risk factors and underlying conditions predisposing to AF are also risk factors for other cardiovascular conditions such as CAD, vascular disease and HF. Targeting these risk factors and underlying conditions as early as possible – ideally before AF becomes clinically manifest – would not only prevent or reverse atrial remodelling and thus prevent or limit AF progression but also improve the underlying conditions themselves and in turn reduce strokes and other cardiovascular adverse events.47–49 This becomes even more important in patients with HF, in whom new-onset AF has a marked effect on mortality compared to patients without HF.50 Moreover, patients with HF who later develop AF have a worse prognosis than those with AF who then develop HF.51
Targeting Risk Factors and Underlying Conditions
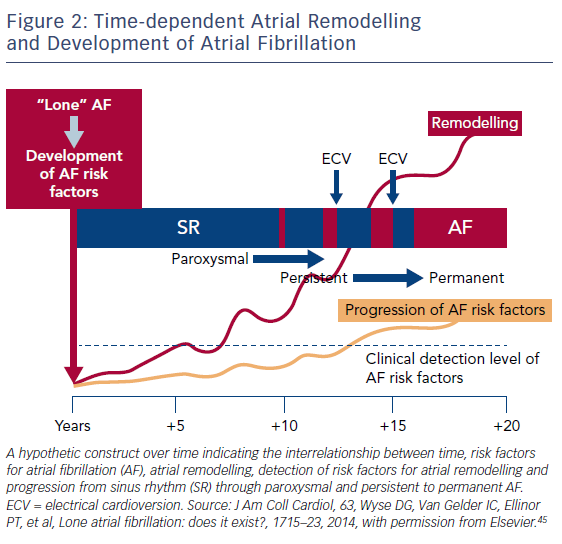
Due to our increasing knowledge about AF aetiologies and mechanisms, there are questions as to whether lone AF exists, as a substantial number of patients who would previously have been classified as having lone AF actually have risk factors (Table 1).45,52 Thoroughly searching for modifiable risk factors and cardiovascular diseases associated with incident AF and initiating treatment as early as possible to prevent or at least delay the development of AF seems prudent (Figure 2).45 For this reason, AF management should also no longer solely address single domains such as stroke prevention, symptom relief or preservation of left ventricular function, but increasingly rely on a broader individual and complete approach with timely detection and optimal treatment of risk factors and underlying conditions to improve outcomes and reduce AF burden by targeting the substrate for AF in a more fundamental way.25,53 As patients with AF have different unfavourable risk factor profiles and many have more than one subclinical or clearly elevated modifiable risk factor,45,54 interventions aiming at risk factor management – including lifestyle modification and treatment and targeting underlying conditions – need to be patient-centred and tailored to individual needs. Thus, targeted therapy of risk factors and underlying conditions has becomes the fourth pillar of integrated AF.25,55 This was recently investigated in the Routine Versus Aggressive Upstream Rhythm Control for Prevention of Early Atrial Fibrillation in Heart Failure (RACE 3) trial.26
Hypertension
Hypertension is one of the major risk factors for AF. The reported prevalence rates of hypertension in AF studies range from 49 to 90 %.56 In the Framingham Heart Study, not only stage II–IV hypertension (systolic blood pressure (BP) >160 mmHg and diastolic BP >95 mmHg) was significantly associated with the risk of AF with an odds ratio (OR) of 1.5 for men and 1.4 for women,57 but also borderline systolic BP was associated with a slightly increased risk of incident AF.7 Data from the Atherosclerotic Risk in Communities (ARIC) study showed that hypertension (systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg and/or treatment for hypertension) accounted for about 22 % of incident AF. The proportion was even higher (24.5 %) if borderline BP values (systolic BP of 120–139 mmHg or diastolic BP of 80–90 mmHg) were included, meaning that even slightly elevated BP is a clear risk factor for AF.54 Similar results were reported from the Women’s Health Initiative (WHI) observational study in postmenopausal women, where an elevated systolic (≥140 mmHg) or diastolic (≥90 mmHg) BP accounted for almost one-third of the population-attributable risk of incident AF.58 In the recently published community-based Prevention of REnal and Vascular ENd-stage Disease (PREVEND) study, use of antihypertensive drugs as a proxy for hypertension more than doubled the risk of incident AF. Likewise, every 10-mmHg increase in systolic BP increased risk for incident AF (HR 1.11).46
The first evidence that optimal treatment of hypertension may prevent AF and improve outcomes came from intervention trials in hypertensive patients. In the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) study, which compared the use of ARB losartan with the beta-blocker atenolol, losartan prevented more cardiovascular morbidity and death than atenolol for a similar reduction in BP.59 A post-hoc analysis from this trial showed that the greatest reduction (40 %) in risk of incident AF occurred in patients who achieved optimal systolic BP levels of <130 mmHg, compared to those with systolic BP ≥142 mmHg. Moreover, incident AF occurred less frequently in patients treated with losartan than in those treated with atenolol, although there was no significant difference in BP reduction.60 A Danish nationwide nested case-control study also found less new-onset AF in patients with hypertension treated with ARBs or ACE inhibitors compared to beta-blockers or diuretics.61 These findings suggest that inhibition of the renin–angiotensin system itself might have a beneficial effect on the reduction of incident AF besides BP control. In a small, randomised study in patients with AF and drug-resistant hypertension undergoing pulmonary vein isolation (PVI) for AF, optimisation of BP treatment by renal denervation on top of PVI significantly reduced AF recurrence at 12 months compared with PVI alone in addition to markedly improved BP.62 A more comprehensive treatment approach was investigated in the RACE 3 trial, where patients with early persistent AF and mild to moderate HF were randomised to causal treatment of AF and HF alone or targeted treatment with mineralocorticoid receptor antagonists (MRAs), statins, ACE inhibitors or ARBs with a BP target of <120/80 mmHg and cardiac rehabilitation on top of causal treatment. Targeted treatment led not only to significantly improved sinus rhythm maintenance but also better BP control at 1 year.26
Heart failure
Beyond age, HF is the most important risk factor for incident AF, increasing the risk by two- to threefold.9,31,57,63–67 Despite this, HF only accounts for a modest proportion of the population-attributable risk of incident AF and has decreased over recent decades, as demonstrated by data from the Framingham Heart Study.7,54,58 These reductions could be ascribed to improvements in HF therapy.
Compared to other risk factors and underlying conditions, HF and AF frequently coexist and have a complex interrelationship. They share many fundamental predisposing factors and pathophysiological pathways, promoting each other and mutually leading to a worse prognosis.50,51,68–70 Data from the PREVEND study have demonstrated that HF is associated with incident AF and that adverse outcomes including HF are associated with AF.46 In daily practice it is often difficult to determine whether AF is a major contributor to shortness of breath, impaired quality of life, clinical signs and worse prognosis or just a coexisting condition. This is because HF – particularly HF with preserved ejection fraction (HFpEF) – and AF share many common clinical signs and symptoms.69,70 Optimisation of HF treatment may prevent AF or at least improve sinus rhythm maintenance. Adding MRA treatment to otherwise optimal HF therapy in patients with mild systolic HF leads to a significant reduction of new-onset AF, as shown in an analysis from the Eplerenone in Mild Patients Hospitalization And Survival Study in Heart Failure (EMPHASIS-HF) trial.71 In addition to improved maintenance of sinus rhythm, comprehensive targeted treatment in the RACE 3 study resulted in improvement of HF, as reflected by a significantly greater decrease in brain natriuretic peptide levels at 1 year compared to baseline.26
Coronary and Vascular Disease
CAD is an established risk factor for incident AF. Data from the Framingham Heart Study demonstrated that a history of MI was significantly associated with incident AF in men (OR 1.4), but not women.57 A later analysis from this study found a significant association when adjusting for age and gender.65 Krahn et al. found a 3.6-fold increase in the relative risk of AF after MI.9 Previous MI was also a predictor of incident AF in elderly patients (mean age 75 years; HR 2.2),63 which was confirmed by the ARIC study66 and in a combined analysis from the ARIC and Cardiovascular Health Studies.64 The PREVEND study also found a significant association between previous MI and stroke and incident AF, with incidence rates of AF comparable to those described in several of the studies mentioned above, although these studies recruited patients much earlier than PREVEND and the treatment of patients with MI and stroke has markedly improved over time. However, incident AF was associated with an increased risk of all forms of vascular disease, HF and death.46 According to data from the Framingham Heart Study, the population-attributable risk of MI remained unchanged over 5 decades despite substantial improvements in the treatment of MI during this time.7 Weijs et al. found a surprisingly high proportion of patients with subclinical CAD in a relatively young (mean age 55 years) cohort of patients with an original diagnosis of lone AF compared to matched controls with sinus rhythm.72 Some of these patients had already developed advanced CAD. Taking into account that patients with AF and vascular disease are at increased risk of fatal and non-fatal cardiovascular events, it seems prudent to screen patients with AF for vascular diseases because treatment in an early stage could reduce AF and improve their prognosis.73 Hypercoagulability may also lead to fibroblast activation, cellular hypertrophy and fibrosis; in this way it may be involved in the creation of a substrate for AF.29,52,74 The contribution of hypercoagulability to the progression of AF is currently being investigated in the RACE V study (ClinicalTrials.gov, NCT03124576).
Obesity
The evidence that obesity is an independent risk factor for incident AF has grown in recent years. Data from the ARIC study showed that overweight and obesity (BMI ≥25 kg/m2) accounted for about 18 % of incident AF, making obesity the second strongest risk factor for AF.54 Comparable results were found in the WHI observational study, where these conditions accounted for 12 % of the population-attributable risk.58 Interestingly, obesity is not only a risk factor for incident AF in postmenopausal women but also in young and essentially healthy women.75 Data from the Framingham Heart Study demonstrated a 4 % increase in AF risk for each unit increase in BMI. Obesity (BMI ≥30 kg/m2) was significantly associated with incident AF in men and women.76 There has been an increase in the population-attributable risk of obesity for incident AF in the past 50 years7 and numerous cohort and case-control studies have confirmed the strong and consistent association between obesity and AF.46,58,77–82 A recent meta-analysis found not only a 29 % and 19 % increase in incident AF risk for every 5 additional BMI units, respectively, but also a 10 % increase in postoperative AF and a 13 % increase in post-ablation AF.83 The PREVEND study found similar results, with an increased rate of incident AF for every 5 additional BMI units.46 Taking these results into account and the fact that overweight is associated with increased risk of fatal and non-fatal coronary heart disease outcomes, it seems prudent to implement fitness and weight reduction in AF therapy.84,85 Cardiac rehabilitation, including regular physical activity, dietary restrictions and scheduled counselling, should be part of a comprehensive targeted treatment approach. In the RACE 3 trial, this approach led to a slight reduction in BMI and weight at 1 year as well as improved sinus rhythm maintenance.26 These figures also demonstrate that a substantial improvement requires long-term patient involvement and persistent adherence to treatment.
Diabetes
Diabetes and elevated blood glucose (BG) levels are also significant risk factors for incident AF, as demonstrated in several studies. However, the results are conflicting and difficult to compare due to differences in methodology, e.g. adjustment for confounding variables which was not performed in all studies. Data from the ARIC study showed that diabetes and poor glycaemic control, reflected by elevated HbA1c levels, were independently associated with an increased risk of incident AF.86 However, another analysis from the same study demonstrated that only 3 % of incident AF was attributable to diabetes.54 The same population-attributable risk was seen in the in WHI observational study.58 The population-attributable risk of diabetes increased over time despite improvements in treatment.7 In a recent Danish nationwide cohort study, the risk for incident AF was most pronounced in diabetes patients aged 18–39 years.87 Poor glycaemic control and longer duration of diabetes were also associated with incident AF in a population-based case-control study that identified a 3 % higher risk of incident AF for each year of diabetes duration.88 In a meta-analysis, individuals with diabetes had 39 % greater risk of incident AF than unaffected individuals.89 Interestingly, AF in patients with diabetes is associated with 61 % greater risk of all-cause mortality and a comparable higher risk of cardiovascular death, stroke and HF.90 The pathophysiological mechanisms implicated in promoting AF in individuals with diabetes are complex and include autonomic, electrical, electromechanical and structural remodelling, oxidative stress, connexin remodelling and glycaemic fluctuations.91 In general, patients with metabolic disorders including diabetes already have an increased risk of fatal and non-fatal coronary heart disease outcomes.84 Taking all these findings together, the vicious combination of AF and diabetes warrants timely evaluation and treatment. In a population-based study, metformin use was associated with a significant reduction in new-onset AF in patients with type 2 diabetes who were not taking other antidiabetic medications.92
Physical Inactivity and Cardiorespiratory Fitness
It is generally accepted that physical activity considerably lowers cardiovascular mortality and morbidity, which is why it is also recommended in current cardiovascular disease prevention guidelines.93 Greater cardiorespiratory fitness (CRF) reduces all-cause mortality and cardiovascular events.94 Results from a large cohort study also showed a graded inverse relationship between CRF and the development of AF, especially in obese patients: every additional metabolic equivalent achieved during exercise testing was associated with a 7 % lower risk of incident AF.95 Similar results were found in a large Swedish cohort study in middle-aged and elderly women that compared self-reported levels of leisure activity. The risk of developing AF decreased with increasing levels of leisure-time exercise at study entry.96 However, the relationship between the amount of exercise and incident AF does not seem to be linear but U-shaped – at least in older adults. This was demonstrated by data from the Cardiovascular Health Study, where individuals doing moderate-intensity exercise developed less AF than those doing high-intensity exercise or no exercise.97 A similar U-shaped relationship has also been found between CRF and incident AF.98 Currently, no exact dose–response relationship has been established between physical activity and reduction of incident AF, but evidence suggests that >220 minutes of moderate-intensity exercise per week or a CRF >8 metabolic equivalents carries a lower risk of AF; whereas high-intensity exercise and endurance training might be harmful and increase this risk.99,100 In practice, routine exercise testing to determine CRF and identify patients at higher risk of AF as well as recommending 150–200 minutes of moderate-intensity exercise per week could be an appropriate solution and should be implemented in the management of AF.85,93,101 This strategy has been investigated as part of a comprehensive targeted treatment approach in the RACE 3 trial, which improved sinus rhythm maintenance.26
Renal Dysfunction
Beyond the established risk factors and conditions associated with AF such as age, hypertension, DM, HF and obesity, renal dysfunction has also been related to incident AF. In the ARIC study, reduced renal function and the presence of albuminuria were strongly associated with incident AF independent of other risk factors.102 Similar results were found in the PREVEND study, where microalbuminuria – as a measure of renal vascular dysfunction – was related to incident AF independent of cardiovascular risk factors.103 Likewise, in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study, renal dysfunction – regardless of severity – was associated with increased prevalence of AF.104 On the other hand, patients with AF have a higher risk of chronic kidney disease, as demonstrated by a large population-based study from the UK and a meta-analysis including approximately 10 million patients from 104 studies.105,106
Reduced renal function is associated with increased risk of adverse cardiovascular outcomes, such as stroke and HF.107,108 It has also been associated with increased risk of stroke and systemic embolism in patients with non-valvular AF.109 The coexistence of both conditions results in a marked increase in both thromboembolic and haemorrhagic risk.110 AF and chronic kidney disease not only share risk factors such as DM, hypertension and HF;111 there is growing evidence that both diseases share underlying pathophysiological mechanisms, such as left ventricular hypertrophy, inflammation, hypercoagulability and activation of the renin–angiotensin–aldosterone system.112–116 Timely treatment of risk factors and underlying conditions could lead to improvement of both conditions and reduce adverse outcomes.
Obstructive Sleep Apnoea
In recent years, obstructive sleep apnoea (OSA) has emerged as one of the novel risk factors for AF.45 Sleep-disordered breathing is a common condition: at least mild OSA affects one in five adults; whereas one in 15 has moderate or severe OSA.117 Moreover, there is a higher prevalence of OSA in men and obese adults, while advancing age and increasing BMI also are risk factors for incident OSA.118 Among patients with AF, the prevalence of OSA is estimated at about 50 % or even higher.119,120 Patients with OSA have a significantly higher risk of developing AF, especially those with severe disease.121,122 A study in patients with OSA and symptomatic AF undergoing AF ablation showed that arrhythmia-free survival was better in those receiving continuous positive air pressure treatment than in those not on this treatment.123 It is important to note that OSA and AF share several characteristics – hypertension, diabetes, obesity and advancing age are common in both conditions. Screening for OSA is regarded as important when evaluating patients with AF, particularly in those with obesity and hypertension. This can be achieved by using simple scoring systems, e.g. the NoSAS score.124
Alcohol Consumption
Acute heavy alcohol consumption has long been known as a cause of AF and is commonly called “holiday heart” syndrome.125 Binge drinking was associated with increased risk of incident AF in an analysis of pooled data from two antihypertensive drug treatment trials.126 Several prospective cohort studies have also looked at the association between chronic alcohol consumption and incident AF. However, the issue with such studies is that, in contrast to other risk factors for AF that can be objectively measured, the quantities of alcohol intake are usually self-reported by the enrolled individuals. Data from the Framingham Heart Study suggested that heavy alcohol consumption of >36 g/day (>3 drinks/day) was associated with a significantly increased risk of incident AF, but also showed that heavy alcohol consumption has decreased over time.7,127 Similar results were reported from the Copenhagen City Heart Study.128 Women who consumed ≥2 drinks/day also had an increased risk of AF, as shown in an analysis from the Women’s Health Study.129 More recently, data form a prospective Swedish cohort study demonstrated that consumption of even small quantities of alcohol was associated with increased risk of AF.130 Furthermore, two meta-analyses showed a linear dose–response relationship between alcohol intake and risk of AF, with a significant 8 % increase in the relative risk of incident AF for each standard drink per day compared to no drinks a day.130,131 These results suggest that there is no safe level of chronic alcohol intake with regard to the development of AF.
Smoking
Numerous cohort studies have investigated the association between smoking and incident AF. Some of them found an increase in risk – ranging from 32 % to more than a doubling in current smokers and 32–49 % in former smokers9,54,132–135 – while other studies did not.77,136–139 An analysis from the ARIC study showed that current smoking accounted for about 10 % of incident AF.54 Moreover, there might also be a dose–response relationship, in that current smokers with the longest duration of smoking and those with the highest number of cigarettes per day had the highest risk of AF.132,133 Generally, smoking cessation is recommended, but data on AF prevention are lacking.
Dyslipidaemia
Current data on the association between dyslipidaemia and incident AF are inconsistent. Unlike ischaemic heart disease, which is clearly associated with elevated LDL cholesterol, it seems that there is an inverse correlation between LDL levels and the development of AF, as shown in several epidemiological studies.138,140–142 Analysis of a pooled dataset from the Multi-ethnic Study of Atherosclerosis (MESA) and the Framingham Heart Study did not find any association between LDL levels and AF, but higher levels of HDL cholesterol and lower levels of triglycerides were associated with lower AF risk.143 The association between higher HDL levels and lower AF risk was also found in two other studies,142,144 whereas no such association was found in an analysis of data from the Women’s Health Study.141 A recent post-hoc analysis from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial showed that patients with AF and higher levels of apolipoprotein A1 had a lower risk of adverse outcomes, i.e. ischaemic stroke, systemic embolism, MI and cardiovascular death, suggesting that interventions increasing HDL levels could have a beneficial effect.145
Data on the effect of lipid-lowering therapy on AF predominantly come from retrospective and small randomised studies investigating statins in patients with post-operative AF (POAF) and their results are mixed.146 A large randomised, controlled trial of rosuvastatin in patients with POAF did not show any beneficial effect of statin treatment in AF prevention.147 Nevertheless, a comprehensive treatment strategy targeting vascular diseases including lipid-lowering therapy with statins could prevent AF progression and improve sinus rhythm maintenance, as shown in the RACE 3 trial.26
Comprehensive Management of Risk Factors and Underlying Conditions
While substantial improvements have been achieved in the field of anticoagulation to reduce stroke and its associated disease burden in patients with AF, important unmet therapeutic needs remain, particularly regarding the prevention of cardiovascular death, HF, unplanned cardiovascular hospitalisations and rhythm control.25,52,148–155 This has led to the concept that “upstream therapy” or “prevention of atrial remodelling” could improve the outcome of rhythm control therapy and possibly also prognosis in patients with AF.30,53,156 Several early retrospective and observational studies on upstream therapy with ACE inhibitors, ARBs and statins have produced encouraging results in terms of reduction in AF recurrences, but larger prospective randomised placebo-controlled trials have failed to show any significant reduction in AF recurrences and adverse cardiovascular outcomes, possibly because these studies only addressed a single risk factor.147,157–161
More recently, evidence has become available that comprehensive interventions that aim to reduce risk factors and underlying conditions of AF are able to reduce AF recurrence and burden in addition to improving the underlying conditions (Table 2). In a small randomised study in overweight or obese patients with symptomatic paroxysmal or persistent AF structured weight management and regular exercise in addition to intensive cardiometabolic risk-factor management led to greater weight reduction, greater reduction of the severity and burden of AF symptoms, and fewer and shorter AF episodes on Holter monitoring compared with general lifestyle advice and cardiometabolic risk-factor management.162 Structured weight management also led to a significant decline in left atrial volumes and pericardial adipose tissue compared with controls.163 Two other non-randomised studies from the same group also showed that aggressive risk-factor management including a structured weight management programme had beneficial effects in terms of AF recurrence, severity and burden of AF symptoms and global well-being in patients with symptomatic AF who were medically managed and underwent catheter ablation for AF.164,165 Importantly, greater weight fluctuations also led to a significantly increased risk of AF recurrence.164 Similar results were reported from a retrospective Italian study in patients with AF – those with higher and increasing BMI had a greater risk of AF recurrence during long-term follow-up.166
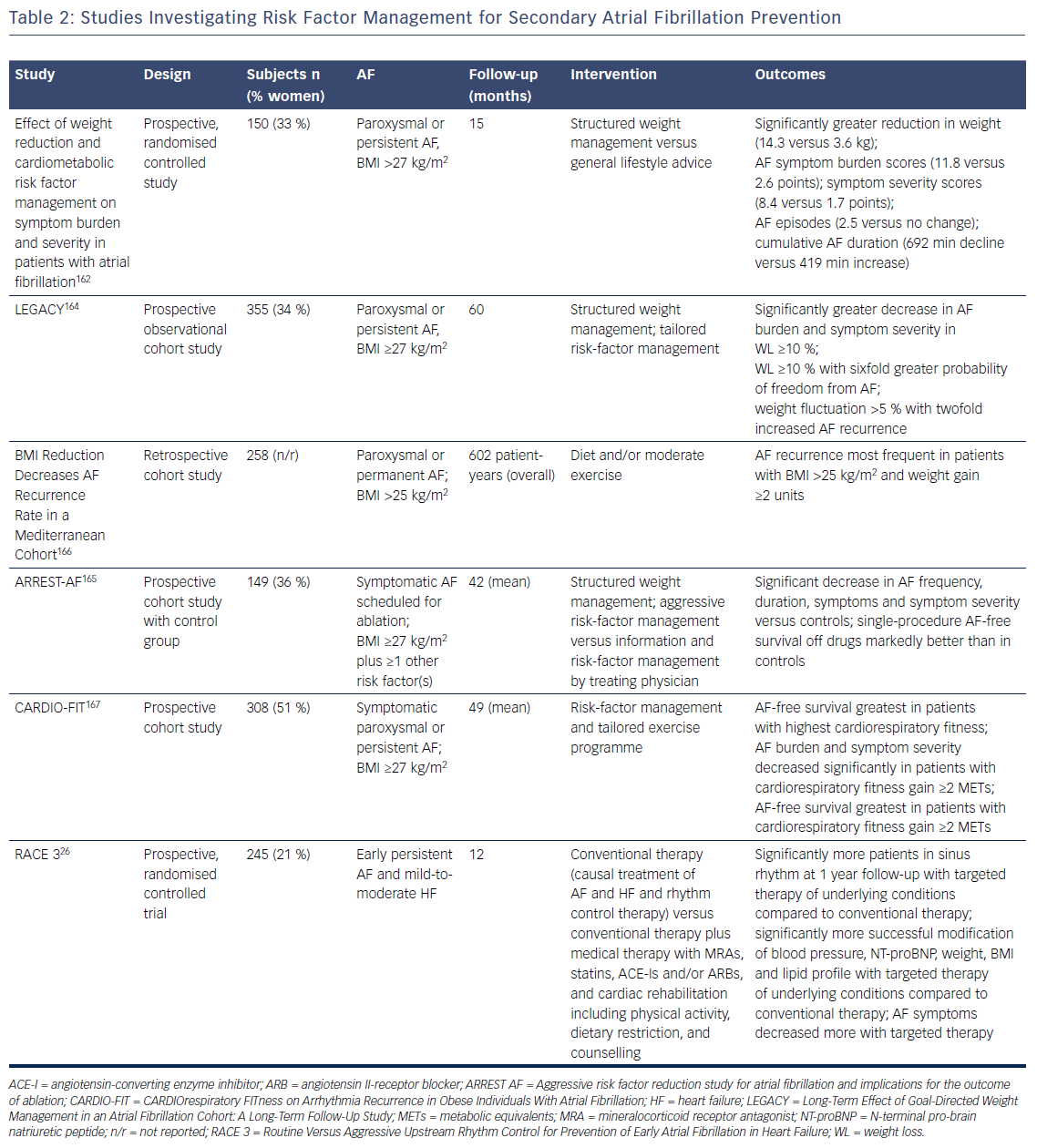
In the CARDIOrespiratory FITness on Arrhythmia Recurrence in Obese Individuals With Atrial Fibrillation (CARDIO-FIT) study, risk factor management and a comprehensive exercise programme led to the greatest reduction in AF recurrence in individuals who had the highest CRF at baseline, and those with the greatest CRF gain and weight loss, suggesting an additional benefit of CRF on top of weight loss.167 Overall, the findings of CARDIO-FIT suggest that fitness might be even more important than weight loss. It is important to recognise that all Australian studies used an aggressive risk-factor management approach in very motivated patients, which might be difficult to apply in daily clinical practice (Table 2).
There is a lack of prospective randomised trials investigating the effect of comprehensive risk-factor management in patients with AF. The RACE 3 trial investigated whether targeted therapy of underlying conditions on top of causal treatment of AF and HF and rhythm control therapy was superior for the prevention of AF in patients with HF compared to causal treatment of AF and HF and rhythm control alone.26 The primary endpoint was sinus rhythm on 7-day Holter monitoring at 12 months. Inclusion criteria were a history of HF <12 months and early symptomatic persistent AF of <6 months duration, not more than one direct current cardioversion, and history of AF <5 years. Targeted therapy included treatment with MRA, statins and ACE inhibitors or ARBs as well as cardiac rehabilitation including supervised physical training two to three times a week, dietary restrictions and counselling.53 At 12 months, significantly more patients in the targeted therapy group had sinus rhythm compared to conventional therapy alone. Moreover, targeted therapy of the underlying conditions led to significantly more successful modification of BP, N-terminal pro-brain natriuretic peptide, weight, BMI and lipid profile. Additionally, AF symptoms assessed by European Heart Rhythm Association symptom score decreased more in the targeted therapy group.
Contemporary Integrated AF Management
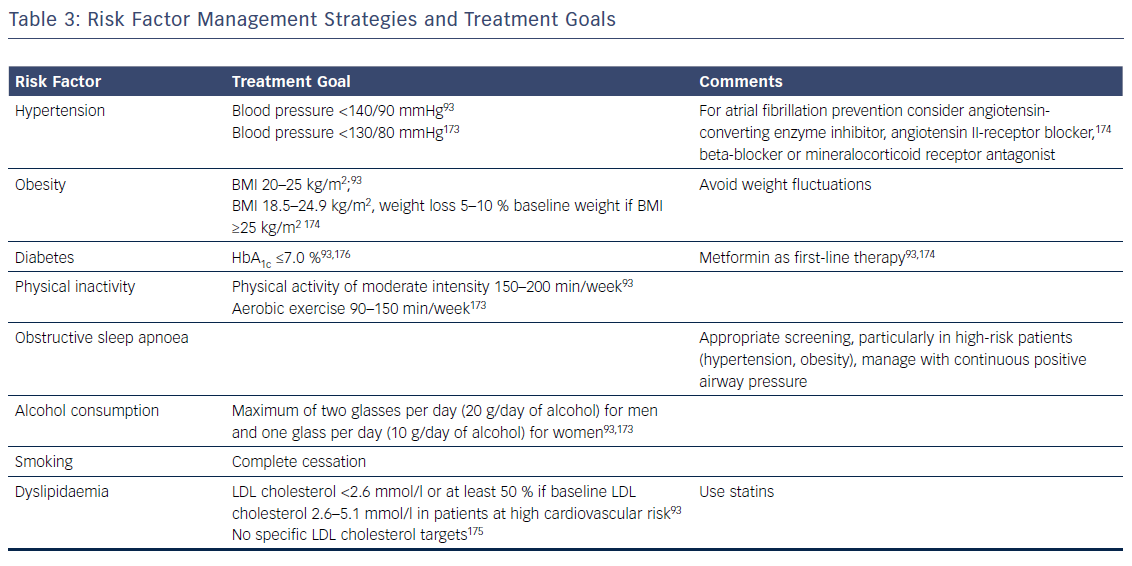
Patients with AF commonly have multiple risk factors and underlying conditions to deal with. This is why AF care becomes increasingly complex and is ideally delivered through an integrated multidisciplinary approach,25,52,151 where medical or invasive treatments and management of risk factors and underlying conditions are tailored and adjusted over time according to the individual needs of patients. As lifestyle interventions and treatment adherence are recognised as being increasingly important, patient involvement in the care process is central in AF management. Key elements of this process are the provision of tailored information about the disease, advice and education on lifestyle modification and risk-factor management, empowerment for self-management, and patient involvement in all treatment decisions, e.g. through shared medical decision-making. Given encouraging data on integrated AF care interventions, a dedicated multidisciplinary AF team or clinic systematically coordinating the patient care and determining individual treatment goals according to current recommendations is key (Table 3).168–171 In the future, early and comprehensive management of risk factors and underlying conditions targeting the substrate of AF together with optimal oral anticoagulation and early targeted and direct treatment of electrical drivers of AF provided by a multidisciplinary AF team could slow progression and improve the outcomes of AF.154,172
Conclusion
Common cardiovascular risk factors – such as hypertension, DM, obesity, OSA, physical inactivity and alcohol consumption – as well as underlying conditions like HF and CAD significantly contribute to the development of AF. Optimal and timely management targeting these conditions is feasible, reduces AF and improves quality of life. However, it remains to be proven whether these interventions also have an impact on other outcomes, such as mortality, cerebrovascular events and cardiovascular hospitalisations.