An estimated 180,000–300,000 sudden cardiac deaths (SCD) occur in the US annually.1,2 Worldwide, sudden and unexpected cardiac death is the most common cause of death,2 accounting for 17 million deaths every year with SCD accounting for 25 % of these. The accepted definition of SCD is death that occurs within one hour of onset of symptoms in witnessed cases, and within 24 hours of last being seen alive when it is unwitnessed.2 The majority of deaths are unwitnessed, with VF being the final underlying mechanism.2–5 The majority of patients are found in asystole or pulseless electrical activity (PEA) and heart block is increasingly noted as an aetiology.
Despite the decline in cardiovascular deaths over the past several decades,6 due to improved preventative strategies, the incidence of SCD as a proportion of overall cardiovascular deaths has increased.2 This has occurred because in-hospital mortality has declined more rapidly,2 highlighting the need for better risk stratification methods and preventative strategies.
Medical therapy with class Ic agents or amiodarone to prevent SCD has been shown to be to be ineffective.7,8 The major advance in the prevention of SCD has been the development of the ICD.9 Secondary prevention trials, Antiarrhythmic Versus Implantable Defibrillator (AVID),10 Canadian Implantable Defibrillator Study (CIDS)11 and Cardiac Arrest Study of Hamburg (CASH),12 have demonstrated statistically significant improvement in survival rates with ICD implantation compared with drug therapy in this patient population.
The Multicenter Automatic Defibrillator Implantation Trial (MADIT)13 and the Multicenter Unsustained Tachycardia Trial (MUSTT)14 studies enrolled patients post MI, comparing primary prevention with an ICD against standard medical therapy in patients with a reduced ejection fraction (EF) (<35 % and <40 %, respectively), plus either documented or induced ventricular tachycardia (VT) and demonstrated a 58–59 % relative risk reduction in death. Subsequently MADIT II15 showed a 28 % relative risk reduction in 2-year mortality in post-MI patients with an EF of <30 % without the requirement for documented or induced VT. The Defibrillators in Non-Ischemic Cardio-myopathy Treatment Evaluation (DEFINITE)16 study compared the benefit of ICD against standard therapy in patients with heart failure, EF ≤35 % and premature ventricular contractions or non-sustained ventricular tachycardia (NSVT), demonstrating a strong trend towards reduced mortality with ICD. While the Sudden Cardiac Death in Heart Failure Trial (SCD-HEFT),17 which enrolled patients with both ischaemic and non-ischaemic cardiomyopathy with New York Heart Association class II or III and EF of ≤35 %, showed a benefit of ICD when comparing ICD with standard medical therapy. What is interesting within these primary prevention trials is that apart from a low EF, no other significant major risk predictors identify who will benefit from an ICD. The major studies have used an EF cut-off between ≤30–40 %; however, the median populations within these studies tend to have far lower EF, and subgroup analysis of patients closer to the cutoff often shows no clear benefit.3,13,15,17,18 Additionally, in the ‘high risk’ population studies, such as MADIT II15 and SCD-HeFT,17 <40 % of patients received appropriate ICD shock therapy during the first 4 years of follow up. Finally, the recent Danish Study to Assess the Efficacy of ICDs in Patients with Non-ischemic Systolic Heart Failure on Mortality (DANISH) showed prophylactic ICD implantation in patients with non-ischaemic cardiomyopathy in patients >68 years was not associated with a reduced long-term mortality.19
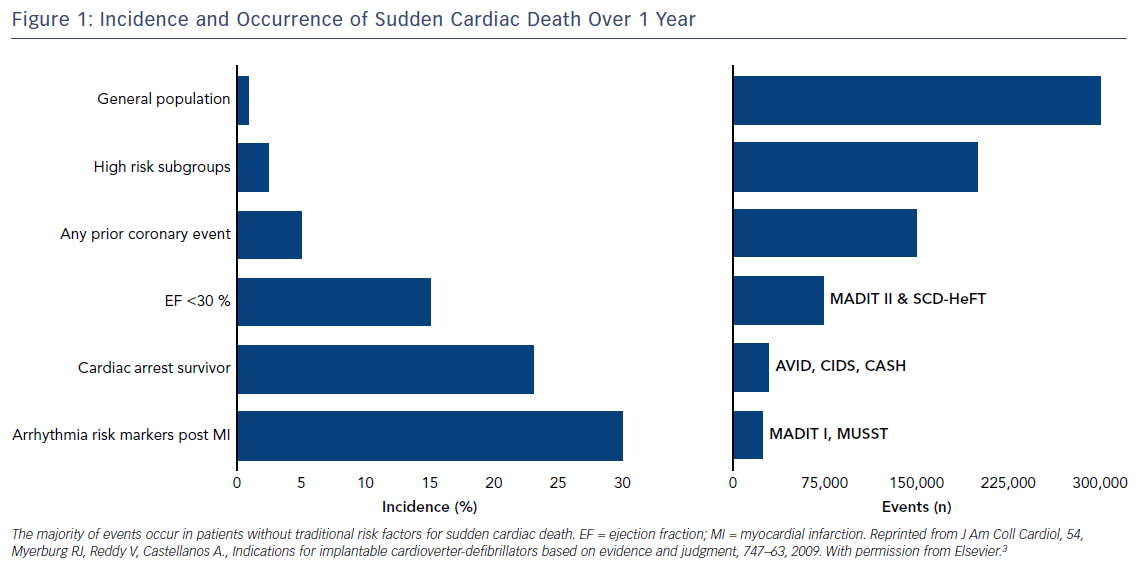
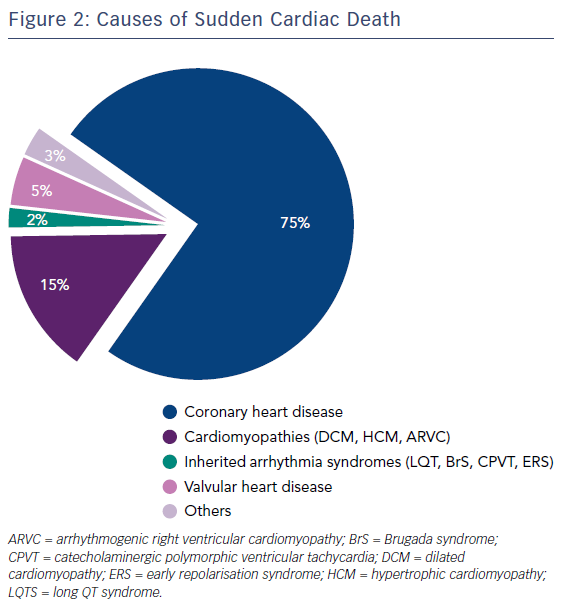
Using these studies to guide prescription of ICDs means that we have only targeted a subgroup of patients where the incidence of events is high and, therefore, they have been labelled as high risk (Figure 1). The challenge for clinicians lies in the fact most episodes of SCD occur in individuals who, prior to the event, have no known cardiac disease and are not perceived high risk by traditional measures, or occurs as a first presentation of an undiagnosed underlying cardiac condition (Figure 2B).3 Thus, the vast majority of SCD events occur in patients who are considered at ‘low risk’ of events.3 Although the incidence within this group of patients is low, they cumulatively account for the greatest number of events (Figure 1). Finally, the indications for primary prevention in less common conditions, such as hypertrophic cardiomyopathy (HCM), arrhythmogenic right ventricular cardiomyopathy (ARVC), long QT syndrome (LQT), Brugada syndrome and early repolarisation, remain less clear, with few if any definite risk markers beyond patient symptoms.3,20
Sudden Cardiac Arrest: Chain of Survival
The majority of cardiac arrests occur outside of hospital, with poor outcomes. In the UK survival to admission may be as low as 8.1 % and survival to discharge as low as 3.2 % for out-of-hospital cardiac arrest.21 The first step to improving outcomes involves the chain of survival:
1. immediate recognition of cardiac arrest and activation of the emergency response system;
2. early CPR with an emphasis on chest compressions;
3. rapid defibrillation;
4. effective advanced life support; and
5. integrated post-cardiac arrest care.
The importance of early recognition and CPR is emphasised by the finding that prompt and immediate bystander response, CPR and early automated defibrillation to an out-of-hospital cardiac arrest by lay members in the community is an important public health initiative that can drive improvements in outcome. Every minute without CPR reduces the chance of survival by 7–10 %. A study of 30,381 cardiac arrests in Sweden found that CPR performed before the arrival of emergency medical services (EMS) more than doubled survival at 30 days.22 Additionally, standardised EMS care, as well as standardised and integrated post-resuscitation care, play a major role in improving outcomes. A study in Oslo where improvements were made to EMS care with an emphasis on uninterrupted and good CPR, followed by goal-directed post-resuscitation care, including therapeutic hypothermia and percutaneous coronary intervention (PCI), doubled survival, with survival as high as 35 % in cases of bystander-witnessed VT/VF arrests.23 In cases of refractory VT/VF, early coronary intervention with extracorporeal membrane oxygenation and uninterrupted mechanical defibrillation during PCI has been shown to produce good outcomes.24 These findings emphasise the importance of a cohesive public health strategy, with public education and engagement in CPR, early access to EMS and focused post-resuscitation care.25
The importance of early bystander defibrillation is emphasised by the fact the early use of public automated defibrillator devices (AEDs) in cases of out-of-hospital cardiac arrest significantly improves outcome.26,27 However, this requires significant political and strategic will to implement, with a need for both widespread and organised access to such devices, as well as public education with regard to their availability. At present, despite campaigns to raise public awareness and make AEDs more publicly available, many public spaces have no available AED. Where they are available, their use may be as low as 1.7 % in out-of-hospital cardiac arrests,28 highlighting the need to re-engage with the public and for better regional planning to close this weak link in the chain of survival, which is the biggest contributor to poor survival rates. New and novel approaches implementing smart technology to detect cardiac arrest and drone networks with automated defibrillators may be the future to improving outcomes.29
Establishing a Diagnosis
Initial Assessment and Management of Patients Who Survive a Cardiac Arrest
Assessment of patients who survive an out-of-hospital cardiac arrest involves a thorough history of the nature of events, including collateral history. The causes of SCD are shown in Figure 2, with ischaemic heart disease being the most common cause. Symptoms prior to the event, such as chest pain, palpitations and breathlessness syncope or pre-syncope, should be assessed. Risk factors, such as hypertension, diabetes, hyperlipidaemia and smoking, should be sought. A drug history should be attained, along with specific questions relating to recreational drug use and use of psychiatric drugs that may prolong the QT interval. Additionally precipitating factors, such as exercise and emotional stress, should be looked for, as well as family history.
The 12-lead ECG remains the hallmark of initial non-invasive evaluation. Where there is evidence of ischaemia, prompt coronary intervention should be performed. However, even in the absence of ischaemic changes on the ECG, up to 29 % of patients with out-of-hospital cardiac arrest will have a culprit lesion, and PCI in this setting is associated with a two-fold outcome in cerebral performance category.30 Thus, all patients should have ischaemia assessed both through the 12-lead ECG and through invasive coronary angiography. The ECG should also be closely analysed for evidence of inherited cardiac conditions and structural cardiac abnormalities. Common inherited cardiac conditions to look for on an ECG include Brugada syndrome, long and short QT and early repolarisation. Additionally, structural cardiac abnormalities, such as HCM, ARVC and dilated cardiomyopathy (DCM), may also present with characteristic ECG changes.
Imaging in the form of echocardiography and MRI play an important role in the assessment of structural causes of out-of-hospital cardiac arrest. Echocardiography is the mainstay of initial assessment, allowing assessment of regional wall motion abnormalities, overall ventricular function, valvular heart disease and heart muscle disorders, such as HCM, ARVC and DCM. Increasingly cardiac MRI (cMRI) is also used to provide additional information to echocardiography, through the ability to provide greater information about tissue wall characterisation, and may contribute to the diagnosis in 50 % of cases and provide a decisive diagnosis in 30 % of cases.31
Patients Where the Cardiac Arrest is Apparently Unexplained: The Barts Protocol
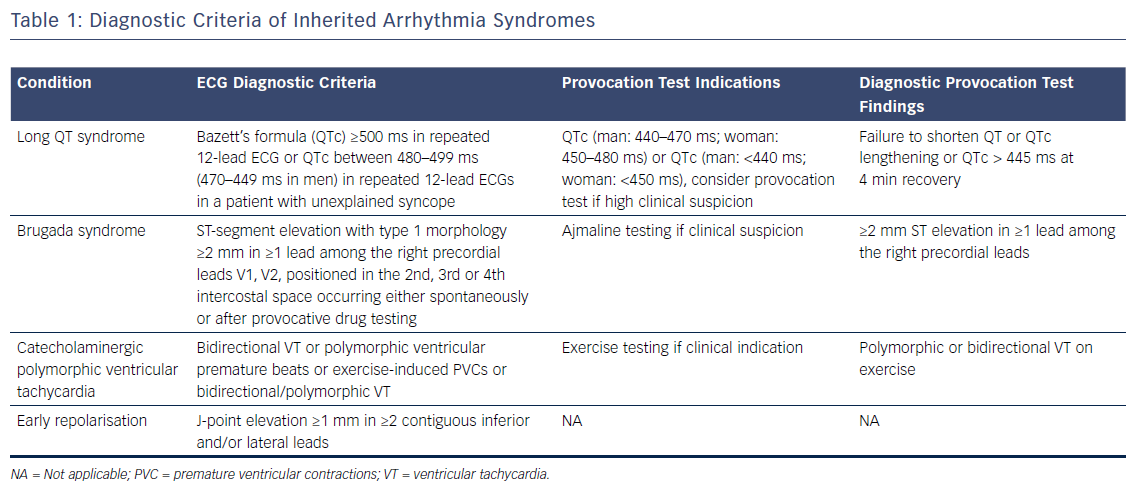
Where cases of ischaemic heart disease are excluded, EF is preserved and repolarisation disorders are not apparent on the resting ECG, further assessment is required. This may increase the diagnostic yield and provide a diagnosis in nearly half of patients where an initial diagnosis is unclear.32 Figure 3 describes the Barts Protocol for Assessment of such patients, and Table 1 details the diagnostic criteria.
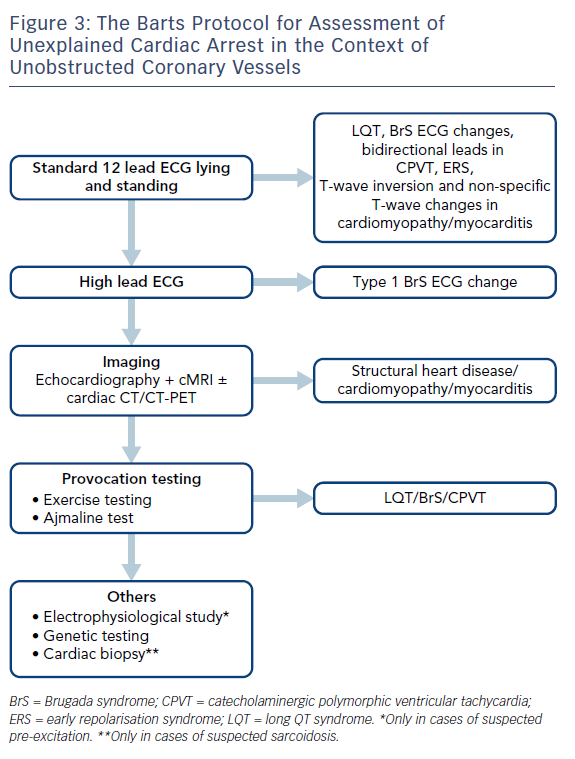
Provocation Testing
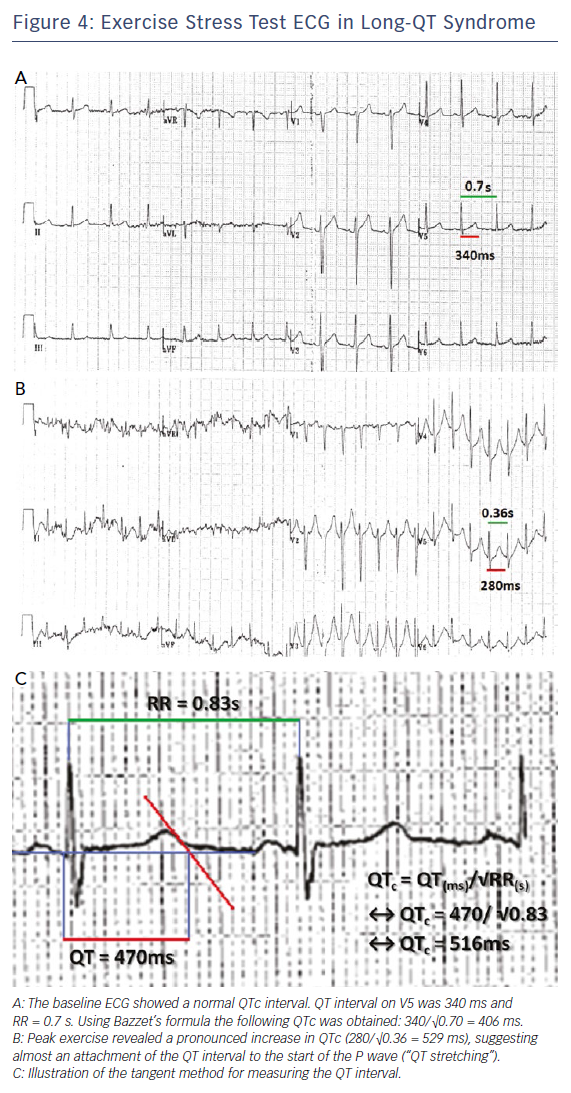
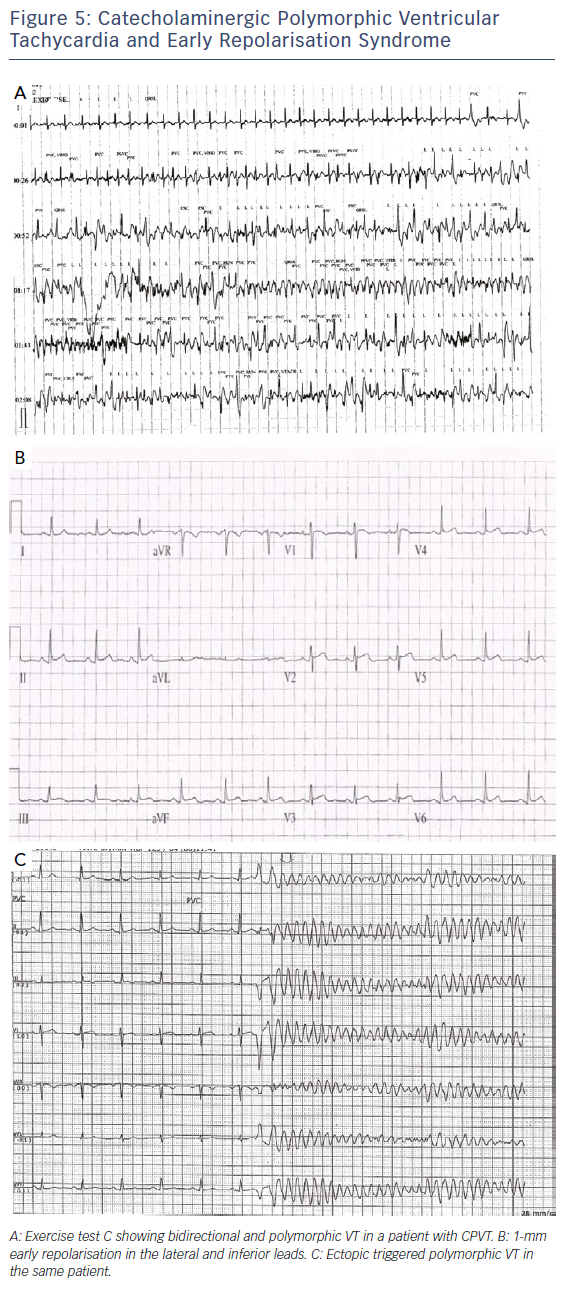
Initial assessment involves a lying and standing ECG to look for evidence of QT prolongation during brief tachycardia induced by standing, which may expose concealed LQT1 and LQT2 phenotypes.33,34 This is followed by exercise testing to look for QT prolongation on exercise or a failure to shorten the QT interval on exercise, which is a prominent feature of the exercise ECG in LQT1 patients.34 Figure 4 shows an example of QT prolongation on exercise along with the tangent method for measuring the QT interval. During treadmill testing particular attention should also be paid to the recovery ECG during the first 1–4 minutes, as LQT2 patients may only exhibit QT prolongation in the recovery phase of exercise where sympathetic withdrawal may provoke late QT lengthening.34,35 A QTc >445 ms at 4 minutes of recovery has been shown to have a sensitivity of 92 % and specificity of 88 % in identifying LQT1 and LQT2 individuals.35 Exercise testing may also expose polymorphic or bidirectional VT, which may hint at a diagnosis of catecholaminergic polymorphic ventricular tachycardia (CPVT) as demonstrated in Figure 5A, though this test is neither sensitive nor specific for the condition.
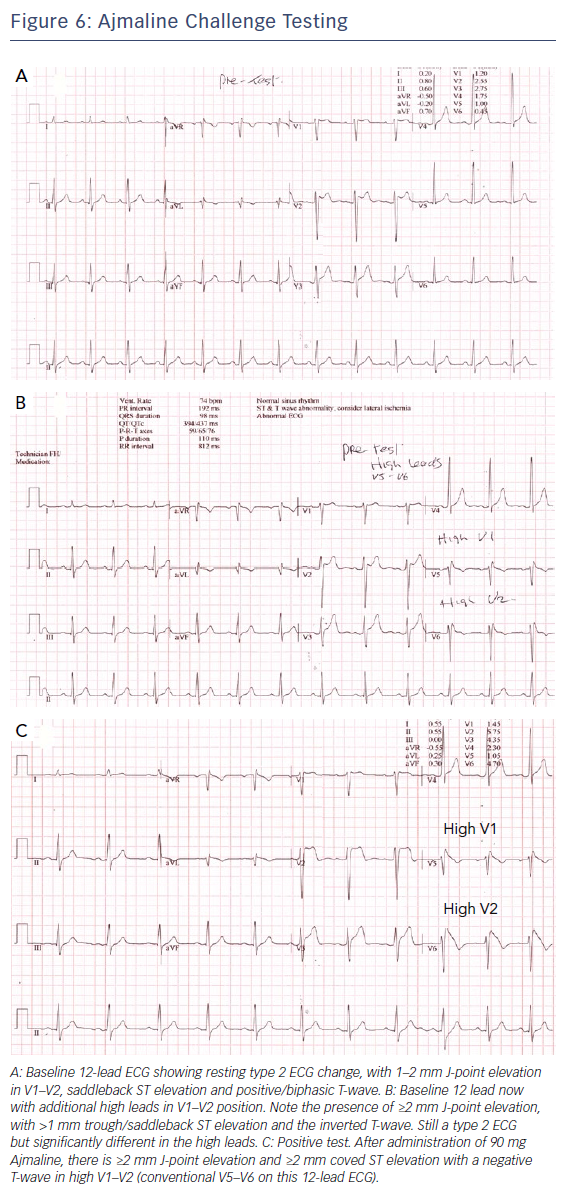
Additionally, we routinely record the ECG in the standard and high-right precordial positions36 to look for characteristic evidence of type 1 Brugada ECG change.37 We also perform provocative testing using ajmaline at a dose of 1 mg/kg over 5 minutes, with the ECG right precordial leads in the high and standard positions, though the sensitivity and specificity of this is debated.36 Figure 6 shows an example of a positive ajmaline challenge test.
Imaging
Echocardiography and cMRI form the mainstay of assessment to look for evidence of structural cardiac causes. cMRI is increasingly proving to be useful through the ability to detect morphological abnormalities and characterise tissue fibrosis, which provides vital clues to the pathogenesis of sudden cardiac arrest.31 Increasingly, where patients do not have a history or ECG changes that warrant immediate coronary intervention, cardiac CT is being used to look at the coronary anatomy and anomalous coronary artery courses, thus negating the need to conventional angiography.
Other Tests/Investigations
Electrophysiological testing has not been shown to influence management or predict outcome in cardiac arrest survivors,38 and its role in management and risk prediction in inherited conditions, such as Brugada syndrome, is debated.39 We only perform this in cases where pre-excitation is suspected. Genetic testing is useful where a clear diagnosis is established or the pre-test probability is high, such that a positive test will influence the management not only of the patient but of their family. Cardiac biopsy may have a role in diagnosing inflammatory or infiltrative diseases, such as myocarditis or sarcoidosis, but is increasingly less used with the advent of advanced imaging modalities, such as cMRI and PET-CT, which we only perform when the imaging findings are in doubt in cases of suspected sarcoidosis.
Management of Patients after Stabilisation from Cardiac Arrest
While implantation of an ICD remains the mainstay of management in most patients who survive an out-of-hospital cardiac arrest, in patients who cannot have an ICD due to clinical reasons or where patients are undecided about having an ICD, the ZOLL® LifeVest may be considered as a bridge to ICD implantation or until the arrhythmic risk subsides.40 In patients who have an ICD implanted, further appropriate ICD therapy is seen in >23 % of cases.32 Identifying the cause of the cardiac arrest is important as this may allow directed therapy that reduces the risk of future cardiac arrest, and may also allow for the identification and prevention of the same, potentially inherited, condition in first-degree relatives.
Ischaemic Cardiomyopathy
Ischaemia is the underlying aetiology in the majority of cases, and arrhythmia management is based on adequate secondary prevention and ensuring adequate revascularisation has occurred. Beta-blockers remain the mainstay of management to prevent further shocks, with additional use of amiodarone or class IB agents, if necessary, to avoid shocks and treat VT storms. Increasingly the use of VT ablation is being performed with success rates >75 % in some cohorts.41
Long QT Syndrome
In patients with LQT, beta-blockers remain the mainstay of treatment, with propranolol, bisoprolol and nadolol being the most effective in shortening the QT interval. Evidence suggestes metoprolol is not as effective.42,43 In patients with LQT3, the addition of oral mexiletine to block late sodium currents may be helpful in patients with a QT interval >500 ms and syncope/pre-syncope or ICD therapy.44 In patients with ongoing ICD therapy despite medical therapy, or with markedly prolonged QT intervals >550 ms where risk becomes higher,20 left cardiac sympathectomy may be effective.45 Additionally, the avoidance of drugs that prolong the QT interval should be emphasised. This list is frequently changing and should be checked by patients and physicians: crediblemeds.org.46
Brugada Syndrome and Early Repolarisation Syndrome
Avoidance of fever, including the use of anti-pyretics and avoidance of drugs that precipitate arrhythmia and type 1 ECG change, is the mainstay of treatment in Brugada syndrome (brugadadrugs.org).47 Acute VT storms respond well to isoproterenol infusion and hydroquinidine can be used as an oral alternative in patients with recurrent ICD therapy.20 Increasingly, epicardial substrate ablation is showing promise48 and may become a major component in patient management.
Early repolarisation syndrome responds in a similar manner to Brugada syndrome, with isoproterenol infusion and hydroquinidine being useful treatments. In some patients with early repolarisation syndrome, VT/VF is triggered by closely coupled ectopic beats (Figure 5B and C), and suppression of these using either beta-blockade, calcium channel blockers or ablation may be useful.
Catecholaminergic Polymorphic Ventricular Tachycardia
Beta-blockers are the first-line drug of choice in preventing ectopy and arrhythmia in this patient cohort. The addition of flecainide is thought to prevent cellular calcium overload in CPVT and may be useful in patients with recurrent syncope, VT or ICD therapy. Verapamil has also been shown to be effective.20 Implantation of an ICD should be avoided in these patients, if possible, because the sympathetic drive associated with a shock can precipitate arrhythmia whether or not the original shock is appropriate or inappropriate. Additionally, there are case reports of ventricular ectopic ablation in CPVT, which may prevent the Purkinje triggers in these patients.49 Finally, left cardiac sympathectomy may be effective in preventing arrhythmia and ICD shocks in cases resistant to medical therapy.45
Idiopathic Ventricular Fibrillation
Management of recurrent arrhythmia in this subset of patients is largely empirical. Recurrence of arrhythmia and appropriate shocks may occur in up to 29 % of patients, and therapy with hydroquinidine50 may be helpful in reducing ICD therapy.51 As with patients with early repolarisation, short coupled ventricular premature beats may be a common arrhythmia trigger in this cohort of patients, often via Purkinje firing, and ablation of these may prove successful in abolishing further arrhythmia.52
Structural Heart Disease
Management of cardiomyopathies is best performed in specialist clinics, where the multitude of symptoms, as well as family screening and follow up, can be performed by physicians with an expertise in the field. Beta-blockers and amiodarone remain the mainstay of treatment for recurrent ventricular arrhythmia in HCM and DCM. Sotolol is the initial drug of choice during the active arrhythmia phase of ARVC, followed by amiodarone or beta-blockers. Catheter ablation may prove useful in reducing arrhythmia burden in ARVC53 and DCM.
Family Screening
Family screening in cases where the diagnosis is clear, or where a clear pathogenic genetic mutation is identified, is necessary to exclude a diagnosis in first-degree relatives, and to manage their risk accordingly. Additionally, screening the family members of victims from unexplained sudden death may identify a disease phenotype that is latent in the proband,54 and may aid in diagnosing and managing the risk to relatives.55 Our screening of family members follows the Barts Protocol.
Conclusion
SCD and arrhythmia continues to represent a major international public health problem and is still the biggest killer worldwide, despite huge improvements in cardiovascular care in the past 30 years. The majority of patients do not survive to hospital discharge, highlighting the need for larger and better public health initiatives to improve the chain of survival. Importantly, the majority of events occur in patients without traditional risk factors for cardiac events, highlighting the need for new and better markers of arrhythmic risk. In patients who survive to arrival at hospital, a thorough assessment of the underlying aetiology is required, and where the diagnosis is unclear, further testing including provocation testing and cMRI is warranted. Management of further arrhythmic events is dependent on the underlying aetiology and screening of family members may aid not only in establishing a diagnosis but also in managing arrhythmic risk of first-degree relatives.