AF is the most common sustained arrhythmia in clinical practice and is increasing worldwide.1,2 AF has been associated with an increased risk of mortality, although data suggest that longevity is increasing after diagnosis. This is attributed to medical therapies to reduce stroke and heart failure, and the management of other comorbidites.3,4 As a consequence, patients are living longer with AF and the detrimental impact of the arrhythmia and its management can been seen with long-term end organ dysfunction.
AF and Dementia Risk With and Without Overt Cerebral Ischaemic Injury
AF is strongly associated with risk of stroke, and patients who experience a stroke have higher rates of progressive cognitive impairment and dementia. Two meta-analyses have shown a composite elevated risk of dementia in patients with AF who have a stroke (RR 2.43–2.70).5,6 AF patients are also two times more likely to experience silent or subclinical strokes than those without AF.7 Silent clinical infarcts are common in AF patients, with MRI revealing these injuries in 40% of patients imaged.7 The premise of a silent infarct is evolving and is likely a misnomer. In AF patients who have subclinical strokes, long-term rates of cognitive dysfunction and dementia are increased compared with those who do not have a stroke.8,9
In an analysis of 37,025 patients, we found that patients with AF had higher rates of multiple forms of dementia, including idiopathic or Alzheimer’s disease, than patients who did not have AF.10 The combined disease state of AF and dementia was significantly associated with mortality (HR 1.38–1.45). Two meta-analyses have evaluated the relationship between AF and incident dementia in patients without clinical stroke or cognitive dysfunction. In this analysis of eight studies, AF was independently associated with increased risk of dementia (HR 1.42; p<0.001).11
Although both dementia and AF are diseases of ageing, two large observational studies found a unique and elevated risk in AF patients who were relatively young (<67–70 years of age).10,12 The association between AF and idiopathic dementia independent of subsequent small or repetitive subclinical strokes is not known. In a subanalysis of the Atherosclerosis Risk In Communities (ARIC) study, cognitive decline was only present in those AF patients who had a subsequent silent cerebral infarct.13 In a study of Alzheimer’s disease patients, MRI imaging of AF patients showed much higher rates of cerebral infarcts and total gray matter volume loss, compared with those who did not have AF.14
The presence of AF in the absence of stroke has also been associated with progressive cognitive dysfunction, without overt dementia. In an analysis from the Cardiovascular Health Study, patients with AF experienced a more rapid decline of cognitive scores – assessed by the Modified Mini Mental State Exam – than those in sinus rhythm (−10.3 versus −6.4 over 5 years for patients with AF and those in sinus rhythm, respectively).15 The risk of cognitive impairment was higher in AF patients with other comorbid diseases such as heart failure, diabetes, kidney disease, etc.16
Worldwide dementia trends show an increased risk in women, particularly in those >80 years of age. Current estimates are that one in two women and one in three men will develop a stroke, dementia or Parkinson’s disease, with the first two diseases comprising the greatest risk.17 We studied 35,608 patients without a history of AF or dementia. In this population 14,377 (40.4%) were women. The 5-year rates of AF were higher in men than women (14.0% in men versus 11.9% in women; p<0.0001), although dementia (1.1% in women versus 0.9% in men; p=0.09) and stroke rates (3.4% in women versus 2.6% in men; p<0.0001) were higher in women. Among the patients who developed AF, the 5-year rate of dementia in women was 2.9% versus 2.3% in men (p=0.180), and the long-term rate was 3.7% in women and 3.0% in men (p=0.110).18
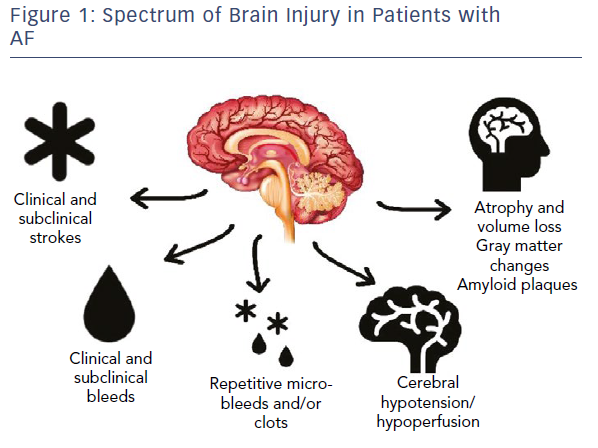
With these emerging data regarding brain injury, it is critical that we start to expand our view of potential brain injuries in patients with AF (Figure 1).
AF and Dementia Risk with Anticoagulation
If macro- and micro-cerebral ischaemic events are significant mechanisms underlying the association of AF with both vascular and idiopathic forms of dementia, then the initiation, use and efficacy of anticoagulation is critical. We studied this concept in an analysis of 2,605 AF patients with no history of dementia or cognitive impairment. These patients were enrolled at warfarin therapy start. This analysis showed that as time in therapeutic range (TTR) is decreased among the categories, the associated dementia risk is increased (versus >75%) (<25% HR 5.34; p<0.0001; 26–50% HR 4.10; p<0.0001; and 51–75% HR 2.57; p=0.001).19 There was a risk of cognitive decline with both over- and under-anticoagulation, suggesting that not only are cerebral ischaemic events a significant risk factor for dementia, but micro- and macro-bleeds also are.
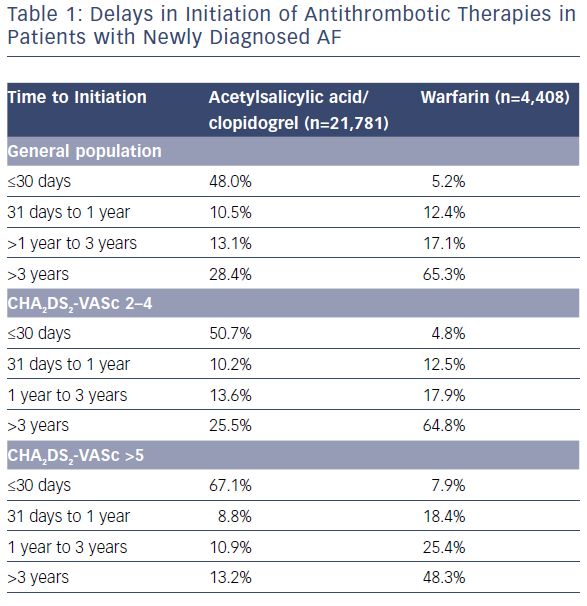
In a more recent national study, 444,106 patients were studied over 1.5 million years of risk. Patients treated with anticoagulation at baseline had a 29% lower risk of dementia than patients without anticoagulant treatment (1.14 versus 1.78 per 100 patient years at risk; p<0.001).20 In this analysis, delays in initiation of anticoagulation had a negative impact on the benefit observed with anticoagulation use (0–1 years HR 0.66; 1–3 years HR 0.80; 3–5 years HR 1.12; >5 years HR 0.80; p<0.001). Unfortunately, underuse and delayed use remain significant issues within our system (Table 1) and worldwide – particularly in women – even in AF patients considered at moderate to high risk.21,22
Direct oral anticoagulants (DOACs) have reduced rates of stroke and intracranial haemorrhage, compared with warfarin.23 In a propensity-based analysis of 5,254 patients (2,627 in the warfarin and DOAC groups), the use of DOACs was associated with a reduced risk of stroke or transient ischaemic attack (p<0.0001), major bleed (p<0.0001), and bleed (p=0.140). In regard to total cerebral events, patients treated with a DOAC were 43% less likely to develop stroke, transient ischaemic attack or dementia than those taking warfarin.24 In a nationwide analysis – limited by the small number of patients who actually received DOAC therapy – use of the newer anticoagulants was associated with a greater relative reduction in dementia risk (HR 0.40) when compared with warfarin.20 A potential benefit of DOAC therapy versus warfarin for brain health and preservation of cognition in AF patients requires prospective evaluation. The Impact of Anticoagulation Therapy on the Cognitive Decline and Dementia in Patients with Non-Valvular Atrial Fibrillation (CAF) trial will evaluate warfarin versus dabigatran over 2 years with serial cognitive testing every 6 months (NCT03061006). As of December 2018, the study was approximately 70% enrolled.
The data regarding anticoagulation use and efficacy are compelling and prompted us to ask if AF contributes to dementia independently in patients treated long term with anticoagulation. In a study of 10,537 patients anticoagulated with warfarin for both AF (n=4,460) and non-AF reasons (thromboembolism n=5,868; valvular heart disease n=209) with no history of dementia, we evaluated the risk of dementia and the potential augmented risk of AF.25 In both groups there was a higher risk of dementia in patients with a low TTR compared with a high TTR, highlighting the critical role of anticoagulation on outcomes. Additionally, in a propensity-based analysis the presence of AF conveyed additional risk for general dementia (HR 2.42; p<0.0001) and Alzheimer’s disease (HR 2.04; p<0.0001).
AF and Dementia Risk Related to Arrhythmia, Haemodynamic Changes and Cranial Perfusion
Many patients with AF experience symptoms of fogginess, mental slowing, or feeling off during transitions from sinus rhythm to AF. The correlation of dynamic cognitive changes with arrhythmia transition cannot be explained by a mechanism of repetitive cerebral injury events from macro- or micro-clots or bleeds.
A compelling mechanism to explain abrupt cognitive decline with AF is that the arrhythmia unmasks cerebral microvascular dysfunction. In an autopsy study of Alzheimer’s disease patients, cerebral atherosclerosis was common.26 The most common area of disease was found in the circle of Willis, which is critical to allow adaptive compensation to all brain regions in the setting of altered blood flow or hypotension. As general vascular risk factors increase, the risk of dementia and the negative relative impact of AF increases.27
A modelling analysis was performed to examine the haemodynamic impact of AF on brain perfusion. In this analysis, variance in R-R intervals coupled with loss of atrioventricular synchrony result in reduced cranial blood flow that causes repetitive hypoperfusions at the arteriolar level and hypertensive events at the capillary level.28 The quantitative impact of repetitive microvascular haemodynamic compromise can be significant in AF patients as another cause of chronic ischaemic injuries and, as such, is considered one of the causes of leukoaraiosis or white matter changes.15
Management of rate and rhythm can improve outcomes. In a small study of patients with persistent AF who were compared with sinus rhythm controls, atrioventricular node ablation resulted in a steady and predictable heart rate (R-R interval), improved frontal and temporal blood flow leading to improved memory and learning and a flow pattern similar to those in sinus rhythm.29 We have also found, in an observational analysis, that patients with AF treated with catheter ablation have lower rates of stroke and dementia than patients who have AF not treated with ablation. Although this finding reflects procedural and referral biases, what was interesting is that patients treated with catheter ablation had stroke and dementia rates similar to patients without a history of AF. For example, Alzheimer’s dementia occurred in 0.2% of the AF ablation patients, compared with 0.9% of the AF no ablation patients and 0.5% of the no AF patients (p<0.0001).30
Risk of Dementia Related to Inflammation, Oxidative Stress and Genetic Components in AF
From a histologic evaluation, Alzheimer’s disease is associated with the accumulation of abnormally folded beta-amyloid and tau proteins that form cerebral plaques. These plaques are associated with cerebral atrophy and cellular death. Amyloid deposits and misfolded proteins are also seen in degenerative atrial myopathy in AF patients.31 Whether the same predisposition of atrial changes associated with amyloid deposits is seen in the brain of patients with AF-related cognitive decline is unknown. From a genetic standpoint the apolipoprotein E epsilon4 allele is associated with risk of dementia and amyloid deposits. However, this allele did not act as a second hit or contributor of accelerated cognitive decline in patients with AF.32
Oxidative stress, inflammation and endothelial dysfunction have been shown to increase the risk of Alzheimer’s disease.33–35 In patients with AF, biomarkers of oxidative stress, inflammation and endothelial dysfunction are elevated.36–38 These markers associated with both disease states suggest both organs that manifest end organ disease – brain (dementia) and heart (AF) – reflect symptoms of a systemic and inflammatory vascular disease that has early roots in hypertension, obesity, low physical activity and metabolic syndrome.27
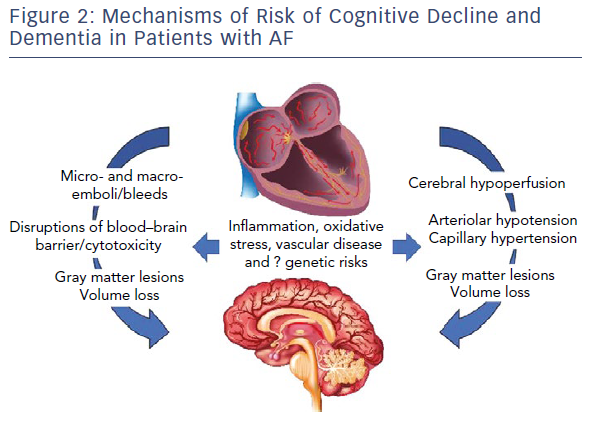
Figure 2 highlights pathways of cranial injury with common mediators that are likely to drive incidence and progression.
Improving Risk Prediction of Cognitive Decline in Patients with AF
Preservation of cognition must be a critical goal in the long-term management of patients with AF. If we consider the concept that chronic cognitive changes reflect repetitive cranial injuries from macro- or micro-clots and bleeds, then traditional risk scores should predict dementia. As discussed previously, the timing, use and efficacy of anticoagulation does influence dementia risk in AF patients. The CHADS2 and CHA2DS2-VASc scores are risk scores used to minimise risk of macro events or clinical events.39 These scores are largely comprised of static baseline risk factors and only augment with time. These scores also do not have the ability to discriminate between the severities of individual diseases, such as a patient with poorly controlled hypertension versus well controlled hypertension. As a consequence, the predictive values of each score are relatively poor, with C-statistics ranging from 0.50–0.70 across multiple cohorts of the study.40 Although these scores also predict dementia risk, there remains broad variability in risk across all CHADS2 and CHA2DS2-VASc strata.27
Risk scores that can be used dynamically and judge severity of disease will likely perform better, so can potentially have an impact on clinical care. For example, a dynamic score can assist in understanding current brain health and risk when transitioning from warfarin with a low TTR to a DOAC therapy, or after lifestyle modifications including increasing activity, lowering weight and improving blood pressure and glycemic control. Risk factor management is complex and multiple factors related to lifestyle drive both AF incidence, progression and adverse outcomes.41
When lifestyle modification is advocated in patients – with the assistance of a multidisciplinary team – arrhythmia-related outcomes can be improved, and some diseases can be reversed. This concept has many potential positive inroads towards cognitive health and the role of adherence to healthy lifestyle choices directed by a multidisciplinary team needs further study.
The sex-specific Intermountain Mortality Risk Scores (IMRS) are a dynamic measurement of systemic health comprised of commonly performed blood tests (complete blood count and basic metabolic profile). These blood tests are used routinely in clinical practice and the individual components used to measure organ and systemic health. The composite IMRS, when separated into three risk categories (low, moderate, high), individually predict risk of incident dementia in both men and women.42 These scores also segregate risk across all CHA2DS2-VASc scores highlighting the value of understanding physiology in the setting of baseline risk.43 The same value of IMRS can be seen in better discerning risk of stroke, as the IMRS improves the C-statistic when combined with CHADS2 and CHA2DS2-VASc scores in both men and women.43
In conclusion, recent guidelines were provided to raise awareness of the critical association between cognitive decline, dementia and cardiovascular disease, to identify key areas of additional research needed and to provide recommendations on how to lower risk.44 In regard to risk in patients with AF, a recommendation for appropriate anticoagulation was made to prevent stroke and cognitive dysfunction. Other areas that may be useful for recommendations include when to consider a DOAC rather than warfarin, optimising TTR to >70%, managing lifestyle changes such as prevention of smoking, hypertension, obesity, diabetes, sleep apnoea, etc., to lower risk of both disorders, and rhythm control – particularly in younger patients (<65 years of age) – which may include ablation in highly-trained centres with long-term follow-up to optimise post-ablation care. 
Clinical Perspective
- AF is associated with long-term risk of cognitive decline and dementia.
- Dementia rates are higher in women and those who have AF.
- Chronic cerebral ischaemic injuries from macro- and micro-clots and bleeds is a mechanism supported by trials of anticoagulation use and cranial imaging.
- Unmasked cerebral vascular dysfunction, through haemodynamic, oxidative and inflammatory mechanisms, is also a probable mechanism that can explain abrupt cognitive changes with onset of AF.
- Several mechanisms of risk can be targeted with current pharmacological and nonpharmacological therapies that may lower dementia risk and, as such, require prospective study.