AF is a common chronic, progressive disease, characterised by exacerbations and remissions. Over the past 10–15 years, multiple large-scale observational studies and randomised controlled trials have demonstrated that catheter ablation is superior to anti-arrhythmic drug (AAD) therapy in maintaining sinus rhythm and improving AF-related symptoms, exercise capacity and quality of life.1–7
The results of catheter ablation can be limited by arrhythmia recurrence, which is most often secondary to a failure to effectuate a durable contiguous circumferential transmural myocardial lesion around the pulmonary veins.6,8,9 In response, considerable effort has been directed towards developing technologies to achieve safer and more durable pulmonary vein isolation (PVI). The two most significant advances in recent years have centred on the integration of real-time quantitative assessment of catheter contact force into focal radiofrequency (RF) ablation catheters, and the development of dedicated catheters capable of achieving PVI with a single ablation lesion (e.g. Arctic Front Cryoballoon, Medtronic).
While the results of the Cryoballoon vs Irrigated Radiofrequency Catheter Ablation: Double Short vs Standard Exposure Duration (CIRCA-DOSE) study are reviewed here, this article focuses on considerations around the design of the study and places the observed outcomes in context.10,11
Study Design
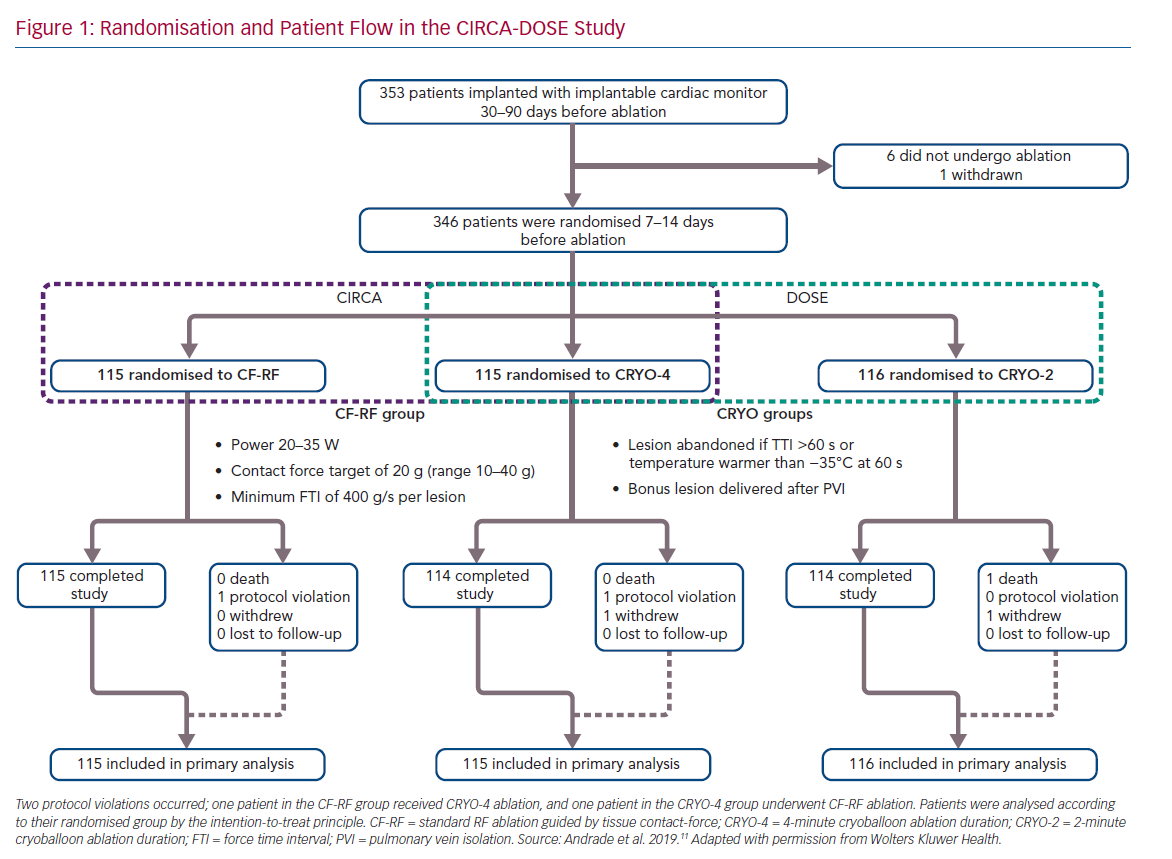
The CIRCA-DOSE study was a pragmatic clinical trial, designed to evaluate two separate clinically relevant questions. The Cryoballoon vs Irrigated Radiofrequency Catheter Ablation (CIRCA) study was designed to compare the effectiveness of PVI performed by advanced generation catheter ablation technologies (contact force guided RF ablation and second-generation cryoballoon-based ablation), and the DOuble Short vs Standard Exposure Duration study (DOSE) was designed to assess optimal cryotherapy dosing. The most cost-effective manner to address these two questions was in the context of a single three-arm trial (Figure 1).
The study was a multicentre, prospective, parallel-group, single-blinded randomised clinical trial, with blinded end-point ascertainment conducted at eight clinical centres in Canada. The study included a total of 346 patients with AF refractory to at least one class I or class III AAD. Patients were randomised in a 1:1:1 ratio to: contact force radiofrequency ablation (CF-RF); short 2-minute cryoballoon ablation duration (CRYO-2); or standard 4-minute cryoballoon ablation duration (CRYO-4). Patients were blinded to their randomisation assignment.
All patients received an implantable cardiac monitor (ICM) for continuous rhythm monitoring, with all arrhythmia events undergoing independent adjudication by a committee blinded to treatment allocation.
Endpoints
The primary endpoint of CIRCA-DOSE was time to the first recurrence of any symptomatic or asymptomatic atrial tachyarrhythmia (AF, atrial flutter [AFL] or atrial tachycardia [AT]) after a single ablation procedure and 90-day blanking period, as detected by continuous cardiac rhythm monitoring. Symptomatic AF/AFL/AT and total atrial arrhythmia burden were considered secondary endpoints.
Rhythm Outcomes
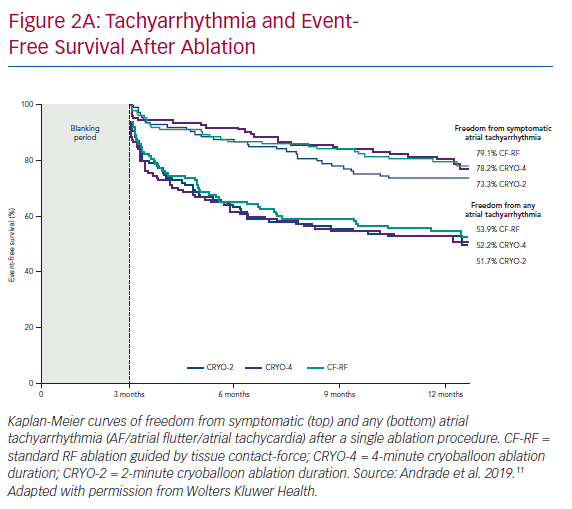
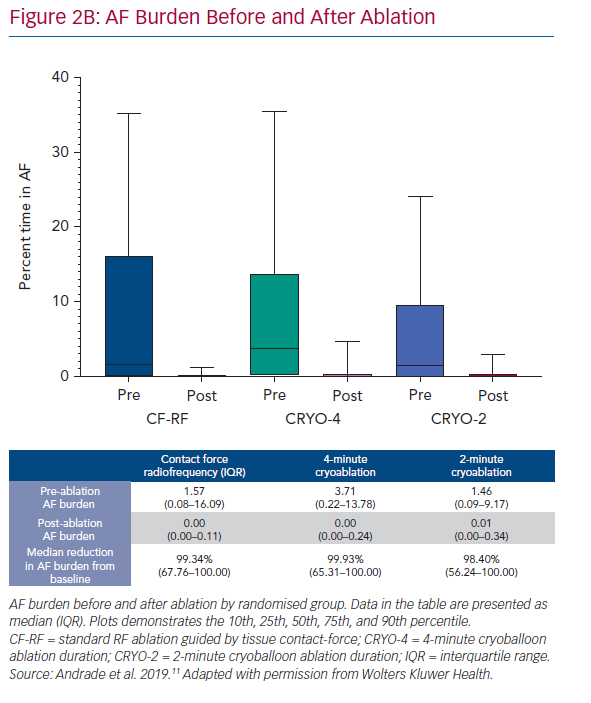
At 12 months, there was no difference in the arrhythmia endpoints between the three randomised groups (Figure 2A). The 1-year freedom from any atrial tachyarrhythmia, measured using continuous rhythm monitoring, was 53.9% with CF-RF, 52.2% with CRYO-4 and 51.7% with CRYO-2; p=0.87. The 1-year freedom from symptomatic atrial tachyarrhythmia, assessed using by continuous rhythm monitoring, was 79.1% with CF-RF, 78.2% with CRYO-4 and 73.3% with CRYO-2; p=0.26. Compared to the pre-ablation monitoring period, the burden or time in AF was reduced by a median of 99.3% (IQR 67.8–100.0%) with CF-RF, 99.9% (IQR: 65.3–100.0%) with CRYO-4, and 98.4% (IQR 56.2–100.0%) with CRYO-2 (p=0.36; Figure 2B).
Safety Outcomes
The absolute rate of complication within the CIRCA-DOSE study was low. Serious adverse events occurred in three patients (2.6%; six events) in the CF-RF arm, six patients (5.2%; seven events) in the CRYO-4 arm, and seven patients (6.0%; 8 events) in the CRYO-2 arm, with no significant difference between the groups (p=0.24).
The most common complication was pericarditis (1.7% overall), with vascular access complications, pericardial effusion, tamponade, thromboembolism and phrenic nerve injury all individually occurring in <1% of patients.
Procedural Outcomes
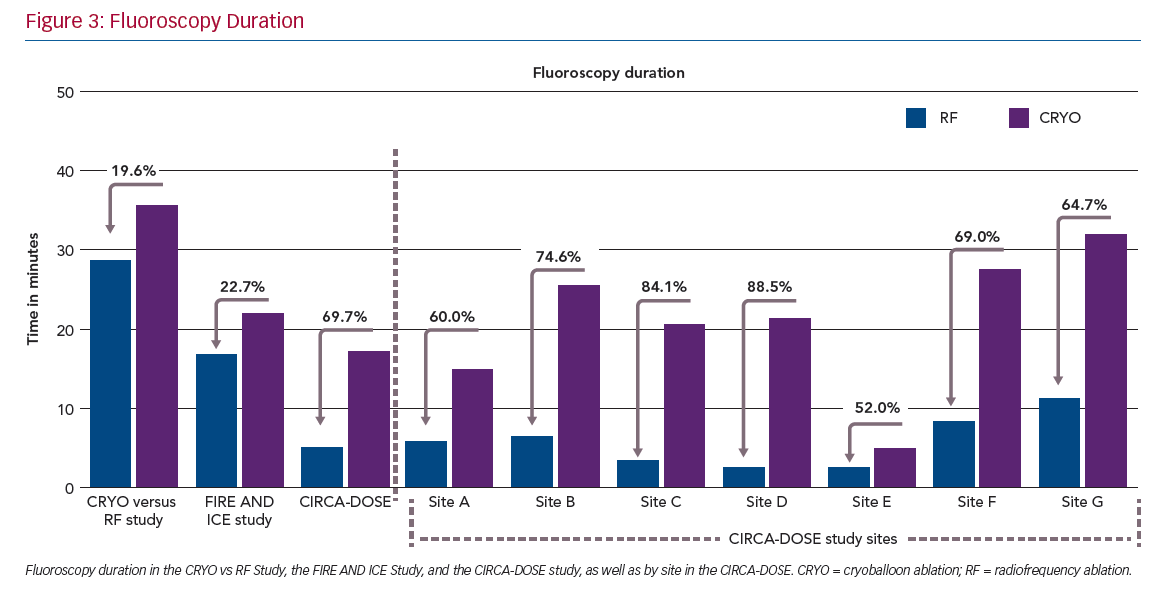
Procedure duration and left atrial access time were both significantly longest in the CF-RF group (164.5 and 143 minutes, respectively), intermediate in the CRYO-4 group (143 and 116.5 minutes, respectively) and shortest in the CRYO-2 group (130.5 and 104.5 minutes, respectively). Conversely, the median total fluoroscopy time was significantly shorter in the CF-RF group than in either of the cryoballoon groups (5.2 minutes with CF-RF versus 17.2 and 19.0 minutes in the CRYO-4 and CRYO-2 groups, respectively).
Quality of Life Outcomes
At baseline, disease-specific and generic health-related quality of life (HRQOL) were moderately to severely impaired. For each of the randomised groups, there was a significant improvement in HRQOL and AF symptom scores at 6 months’ follow-up. This improvement was sustained at 12 months.
What does the CIRCA-DOSE Study Tell Us?
First and foremost, the CIRCA-DOSE study convincingly demonstrates the efficacy of PVI for patients with paroxysmal AF. The observed substantial (>99%) reduction in AF burden was significant and clinically relevant, given that the major indication for PVI is to alleviate symptoms associated with recurrent arrhythmia episodes. Second, the study demonstrated that these outcomes were consistent across the evaluated technologies. Specifically, there was no significant difference between contact-force RF ablation and cryoballoon ablation with respect to recurrence of any atrial tachyarrhythmia, symptomatic atrial tachyarrhythmia, asymptomatic AF, symptomatic AF or AF burden. Similarly, there was no difference in the frequency of complications between randomised groups. Finally, the 53% time-to-first recurrence success rate corresponded to a >99% AF burden reduction, suggesting that the use of a binary time-to-event outcome may underestimate clinical benefits to patients.
How Do the Results Compare to Other Contact Force Radiofrequency Ablation Studies?
In contrast to the two largest multicentre trials evaluating contact-force RF technology (TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation [TOCCASTAR] and THERMOCOOL® SMARTTOUCH™ Catheter for the Treatment of Symptomatic Paroxysmal Atrial Fibrillation [SMART-AF]) the CIRCA-DOSE study protocol pre-specified the contact force and lesion delivery parameters.12,13 For CIRCA-DOSE, the pre-specified target contact force of 20 g (acceptable range 10–40 g) with a minimum individual target lesion duration of 400 gram-seconds force-time integral (FTI) was based on data derived from the TactiCath® Prospective Effectiveness Pilot Study (EFFICAS I and II) and Touch+™ for Catheter Ablation (TOCCATA) studies, which were the best data available when the CIRCA-DOSE study was initiated.14–17
Within this context, the observed freedom from symptomatic AF/AT/AFL of 79.1% in the CF-RF group in CIRCA-DOSE is numerically greater than that observed in the SMART-AF and TOCCASTAR trials (72.5%, and 66% respectively).12,13 However, the results in CIRCA-DOSE are comparable to those observed in SMART-AF when operators were working in their selected CF-ranges ≥80% of the time.
While it is possible that the high success in CIRCA-DOSE reflects the value of prospectively targeting pre-specified CF parameters such as FTI, it is difficult to know how these results may have differed with the use of novel ablation lesion quality markers such as ablation index, which incorporates contact force, time and power into a weighted formula.6,13,18
Why Was Any Atrial Tachyarrhythmia the Primary Endpoint?
Given that the CIRCA-DOSE study was designed as a comparative technology evaluation, we felt that a determination of the true efficacy of AF ablation required the inclusion of both symptomatic and asymptomatic arrhythmia episodes, as detected by continuous rhythm monitoring. While freedom from AF-related symptoms may be the most important endpoint from a patient perspective, contemporary evidence suggests a poor correlation between symptoms and AF burden.19,20 Moreover, studies reporting ablation success based on freedom from symptomatic arrhythmia have a tendency to overestimate treatment success (defined by the absence of detected AF) by 20% or more, which is consistent with the difference observed in CIRCA-DOSE (i.e., a 21.6–26.0% difference between symptomatic and any AF/AFL/AT).2,6,12,13,18,21,22
Including AT and AFL within the primary endpoint recognises that iatrogenic tachyarrhythmias can be caused by incomplete scar formation secondary to the ablation procedure itself. This is relevant to our study design because previous studies have suggested that CF-RF may be associated with a higher rate of atypical flutter/tachycardia than cryoballoon ablation.23
Finally, while some studies permitted multiple ablation procedures within the periprocedural blanking period “without penalty with regard to the primary efficacy endpoint”, we felt this would overestimate treatment success.3,12,13,24 As such, we considered a repeat ablation procedure at any time to constitute a treatment failure.
Why Were Implantable Cardiac Monitors Used to Document Arrhythmia Recurrence?
While non-invasive intermittent rhythm monitoring remains the most widely used method of ascertaining ablation efficacy, it lacks sensitivity in detecting sporadic arrhythmias such as paroxysmal AF. This would be expected to result in recurrences being underdetected, inflating estimates of arrhythmia-free survival and introducing misclassification errors that could potentially impact the accuracy and precision of comparative risk estimates.25–27 This imprecision associated with intermittent arrhythmia monitoring confers a significant risk of a type II error, which makes non-invasive intermittent rhythm monitoring inappropriate for outcome ascertainment in a trial designed to evaluate the efficacy of different therapeutic interventions.
In addition to optimising arrhythmia detection, the use of an ICM optimises adherence through automated home monitoring (CareLink, Medtronic). For the reasons set out above, non-adherence to rhythm monitoring protocols can result in under-detection of recurrences, and consequent misclassification errors. Unfortunately, compliance with non-invasive rhythm monitoring has been reported to be as low as 59% in contemporary AF ablation studies.28
Finally, using an ICM provided an accurate quantification of AF burden or the proportion of time an individual is in AF.22,25,26,29 While time to first AF recurrence has been considered the gold standard for reporting the efficacy of AF ablation procedures, these dichotomous endpoints considers AF as a binary condition, which provides an incomplete assessment of ablation outcomes.22,29 Virtually no other chronic cardiac condition, such as heart failure or angina, is considered in similar binary terms. In evaluating AF burden with ICMs, we were able to consider AF as a quantitative entity on a continuous scale, which provides a more comprehensive evaluation of treatment effect of ablation.22
Why Were 2-minute Freezes Chosen for the Short Duration Group?
The optimal duration of freezing – how long the tissue should be kept in a frozen state – is not well established. The previous recommendation was for cryoablation dosing at 240 seconds for each application. This recommendation was derived from studies of an early focal cryocatheter. In these early cryofocal studies, it was observed that the effect of a cryoablation lesion reached a plateau of 3 minutes after the onset of ablation. Thereafter, “prolongation of exposure time beyond 3 minutes did not result in any further increase in lesion dimension or volume”.30,31 Since then, the cryocatheter has evolved from a rigid focal catheter to a semicompliant balloon, which meant the cryorefrigerant delivery mechanisms had to be redesigned. Moreover, the refrigerant itself has changed from slow-cooling to more efficacious gases.
Before undertaking the CIRCA-DOSE clinical study, we completed two randomised pre-clinical studies examining the immediate and delayed effects of shorter cryoablation times.
In the first study, a focal cryocatheter was used. This study demonstrated no difference in ablation lesion surface area (167.8 ± 21.6 mm2 versus 194.3 ± 22.6 mm2; p=0.40), maximum depth (4.4 ± 0.2 mm versus 4.5 ± 0.2 mm; p=0.71) and volume (125.7 ± 69.5 mm3 versus 141.0 ± 83.5 mm3; p=0.25) between 2-minute and 4-minute freezes, as assessed by three-dimensional morphometric analyses.32 This study concluded that single 2-minute and 4-minute applications result in catheter ablation lesions of a similar size using the modern cryoablation system with nitrous oxide as a refrigerant.
The second study examined a single 2- versus 4-minute cryoballoon application, specifically assessing PVI efficacy.33 In this study, 32 dogs underwent cryoballoon ablation with a 23 mm cryoballoon catheter. PVI procedures were randomised to a 2-minute versus a 4-minute cryoballoon application. Although 4-minute lesions were associated with a thicker neointima than 2-minute lesions (223.8 μm versus 135.6 μm; p=0.007), no differences were observed in the rates of procedural PVI or in the achievement of complete circumferentially transmural lesions at 30 days (78% overall; 86.2% for 2-minute versus 70% for 4-minute lesions; p=0.285). However, fewer late pulmonary vein (PV) strictures were observed in the 2-minute group (6/30 PVs with strictures in the 4-minute freeze duration versus 0/29 PVs with strictures in the 2-minute freeze duration; p=0.024). As such, the CIRCA-DOSE study was designed to evaluate whether an abbreviated 2-minute cryoablation lesion would have a similar efficacy to a cryoablation lesion of a standard duration.
While clinical practice has moved to 3-minute cryoablation durations, it is important to recognise that the evidence supporting the use of a 3-minute lesion is derived from non-randomised studies, where 3-minute cryoablation durations were compared to historical controls.34,35 Even though we did not specifically study 3-minute cryoapplications, it is reasonable to interpret the results of the CIRCA-DOSE study in supporting the use of a 3-minute cryolesion, given that the study demonstrated similar efficacy in the primary endpoint for both the 2-minute and 4-minute cryoablation groups.
Why Were Time to Isolation and Time to Effect Not Used?
Time to isolation (TTI) and time to effect (TTE) are physiological intra-procedural predictors of lesion durability, with a short time to effect associated with a higher likelihood of delivering an efficacious lesion.36 As TTI can be evaluated in real time during the index ablation procedure, there has been a recent trend for protocols to incorporate assessment of real-time PVI into cryoablation dose-titration algorithms.37
While the CIRCA-DOSE study did not explicitly titrate the cryoablation lesion duration on the basis of the observed TTI, the study did prospectively incorporate TTI assessment into the cryoablation protocol. Specifically, a key focus of the study was on determining if shorter cryoballoon ablation durations were as efficacious as standard cryoablation durations. As the permanence of cryoablation lesions are a function of catheter tissue contact, tissue temperature and freezing duration, we were concerned that late PVI (occurring >60 seconds after freezing onset) would disproportionately affect the short cryoablation group (CRYO-2). To avoid this, we prospectively mandated that lesions that fail to isolate the vein after 60 seconds of ablation onset be terminated. In patients where real-time PV monitoring could not be achieved, we employed a temperature cut-off of −35°C at 60 seconds as a surrogate for lesion efficacy. This combination ensured that the lesion would be as efficacious as possible.
How do Patients in CIRCA-DOSE Compare to Others?
The CIRCA-DOSE study enrolled patients with highly symptomatic paroxysmal AF refractory to AADs. The patients included in the study reflected those in contemporary AF ablation trials, with the majority being men in their late 50s with normal atrial dimensions and a low CHA2DS2-VASc score.38 Two-thirds of patients were classified as having a Canadian Cardiovascular Society Severity of AF score of 3 or more, signifying that the symptoms attributable to AF were having a moderate to severe impact on their general quality of life. Likewise, both the disease-specific (AFEQT) and generic (EQ-5D) HRQOL scores were moderate-severely impaired at baseline (mean Atrial Fibrillation Effect on QualiTy-of-Life [AFEQT] score 54.2; mean EQ-5D score 0.867).
The pre-ablation AF burden recorded on continuous monitoring was 1.5–3.7%, which corresponds to 1 hour of AF every 1–3 days. Unfortunately, it is difficult to contextualise this burden, as CIRCA-DOSE is unique as continuous cardiac rhythm was monitored in the months before the ablation procedure. Using more commonly reported surrogates of pre-ablation AF burden, the median of 4.0 AF episodes per month in CIRCA-DOSE and the observation that patients had failed a median of 2.0 AADs before enrolment are both comparable to contemporary studies.1,3,4,6,18,21,38,39
How Can We Interpret the Procedural Data?
Consistent with the previous randomised comparisons, we observed a significantly shorter procedure duration but significantly longer fluoroscopy duration with cryoballoon ablation. While these results are consistent on the whole with previous randomised comparisons, it is important to note that the fluoroscopy time in CIRCA-DOSE was lower than that reported in previous comparative studies (Figure 3). Conversely, while the procedure time was longer in CIRCA-DOSE, this was likely to be a function of the mandated 20-minute post-ablation observation period in combination with the administration of adenosine for the assessment, as per protocol, and subsequent elimination of dormant conduction.
Interestingly, when the analysis was stratified by site, these differences became more significant. This suggests that the between-site variability resulted in significant overlap when the results were considered only by the randomised groups. Within each site, there were consistent and substantial differences in procedural and fluoroscopy times between these ablation technologies. In all study sites, the procedures with cryoablation were significantly shorter, and procedures with CF-RF used less fluoroscopy.
What Else Can We Take Away?
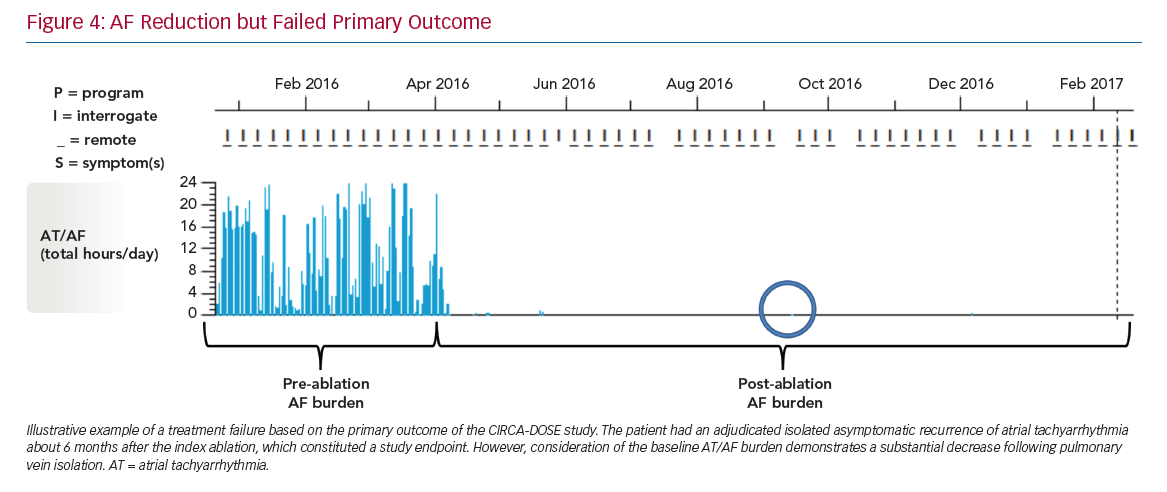
The stark contrast between the rates of the primary endpoint (~53% success when based on AF recurrence as a dichotomous outcome) and the magnitude of reduction in AF burden (~99% compared to pre-ablation monitoring) highlights the need to reappraise the optimal endpoint for establishing the success of AF ablation procedures (Figure 4).
Conclusion
The CIRCA-DOSE trial demonstrates that PVI performed by cryoballoon ablation or by contact-force guided RF ablation results in comparable freedom from recurrent atrial tachyarrhythmia as assessed by continuous cardiac rhythm monitoring. Binary efficacy outcomes, such as AF recurrence, appear to underestimate the treatment effect of AF ablation.
Clinical Perspective
- Arrhythmia outcomes are comparable after pulmonary vein isolation using the cryoballoon ablation and contact force-guided radiofrequency energy, suggesting that either procedure can be performed depending on operator preference and skill.
- Shorter cryoballoon ablation durations (freezing for 2 minutes instead of 4 minutes) significantly reduce procedural duration without compromising arrhythmia efficacy.
- Binary arrhythmia efficacy outcomes underestimate the clinical impact of catheter ablation, with a 53% time-to-first-recurrence success rate corresponding to a >99% AF burden reduction.