Dr O’Donnell began by stating that the response to CRT is suboptimal and a significant proportion of the poor response to CRT is due to poor positioning of the LV leads. In a typical left bundle branch block (LBBB), the response to routine CRT is 60 to 70 %, with an 8 % improvement in EF. Most physicians consider this a good response rate. However, if CRT is electrically mapped, outcomes can be further improved to yield an 80 % response rate and 12 % improvement in EF.
In order to improve CRT outcomes, we need to consider the factors that influence the response. These include patient selection: an increasingly smaller group are becoming eligible for CRT, meaning that a significant number of patients with HF are missing out on a treatment that may benefit them. Prior to electrical mapping, patients with atypical LBBB had a poor chance of responding to CRT. Randomised studies show response rates of around 40 % and EF improvements of 3%. Electrically mapped CRT nearly doubles the response in these patients (response rate 75 % and an 11 % improvement in EF).6
Patients with other conduction abnormalities, including a narrow QRS, right bundle branch block (RBBB) and non-specific IVCDs (NIVCD) have also typically been excluded from CRT. Many consider that traditional CRT is ineffective for these patients; in cases of narrow QRS, response rate is negligible with no improvement in EF.7 However, with electrically mapped CRT, the response rate is 65 % and the EF improves by 8 %. The same is true of RBBB/NIVCD, where electrically mapped CRT can give responses of 60 % with an EF improvement of 6 %.8
Correct lead placement is also essential to optimise responses to CRT. Dr O’Donnell explained the challenges of optimising myocardial cell-to-cell transmission in CRT using the analogy of transmitting a message to a group of soldiers in such a way that each soldier receives the message as quickly as possible. However, CRT presents unique challenges such as scar (some soldiers do not pass on the message); conduction block (some soldiers only pass the message in one direction); functional block (some soldiers pass the message slowly or incompletely); and intrinsic conduction (some soldiers utilise intrinsic communication systems).
In other words, we need to focus on where to put the leads, the electrical conduction and the electrical map. In the same way that golf clubs and tennis racquets have a ‘sweet spot’ for an optimal shot, Dr O’Donnell suggested that there is a sweet spot in CRT. However, at present, we do not know where or how big it is. The remarkable improvement experienced by some patients with anterior leads suggests that in some people, the sweet spot is clearly bigger than others and in a broad left bundle with notching, it may be 2 to 3 cm, and the lead positioning is less crucial; whereas in cases of narrow QRS, it may be as small as 2 mm. This raises the questions: how close do we have to place the leads to achieve 90 % of the effect? If we do not find the right spot, are we making these patients worse? Large studies suggest that 10 to 15 % of patients deteriorate following CRT.9
Dr O’Donnell presented his ideas as a series of concepts. Concept 1 is that there are better and there are worse places to pace in the heart. Others argue that this is incorrect and support their arguments with data that show the LV lead location does not impact on mortality or measures of response to CRT.10 Interpretations of the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT) data have also led to the conclusion that the lead position is unimportant.11 However, this study concluded that, anatomically, it is difficult to determine where to put the lead, but areas of scar or the apical region should be avoided. This leads to Concept 2: anatomical placement is ineffective for finding the sweet spot. Therefore, if the sweet spot exists, it must be electrical. The reasons that anatomical placement fails is evident if we look at the different forms of septal activation in LBBB – a slide was presented of 20 different anatomical specimens of LBBB in 20 different patients – so it is unreasonable to expect that placing the lead in the same position in everyone will be beneficial.
A study of electrical activation in LBBB shows that there are multiple types of activation, and most people consider the typical LB that travels down the septum in a U shape but this activation pattern occurs in less than 50 % of all LBBB.12 A recent study of patients with LBBB found that the type of bundle block significantly influences response to CRT; an absence of ECG markers of residual LB conduction was predictive of a greater improvement in LV function with CRT.6
A study found that markers of electrical dyssynchrony, including the electrical delay at the LV pacing site (QLV) are predictive of response to CRT. Activation was measured as the time that the intrinsic electrical wave front detected in the RV lead takes to travel to the LV lead,8 a simple process that takes only seconds. If RV and LV electrograms are on time with each other, it is difficult to resynchronise the entire area effectively. Such patients are termed non-responders. In a typical non-responder, intrinsic RV–LV = 20 ms, QRSd = 145 ms, QLV = 15 %. In a typical responder intrinsic RV–LV = 120 ms, QRSd = 145 ms, QLV = 82 %, i.e., a large electrical delay. There was moderate correlation between intrinsic RV–LV electrical delay and delta LV versus absolute change in LVEF (p=0.03).8
Many groups have validated this study. Electrical delay in the LV lead has been shown to be correlated with the haemodynamic response to CRT expressed as an intra-individual percentage change in the maximum rate of rise of LV pressure over baseline (dP/dt, derived from the mitral regurgitation Doppler profile).13–15 Later activated areas are associated with higher stroke volume16 and improved LV synchrony as determined by tissue Doppler imaging (TDI).17 The seminal electrical mapping study by Gold et al. found that a measured QLV interval is an electrical marker of delayed LV lead position and that long QLV is associated with better echocardiogram and clinical outcomes.18 The LV end-systolic volume and quality of life (QOL) response rates were statistically significant in the QLV quartiles used.
Another study examined the impact of electrical and anatomical location of the LV lead in relation to baseline QRS morphology on the CRT outcome.19 The best response was seen in a patient with an LB and an LV lead late in the QRS complex. Interestingly, patients with a non-left bundle and a narrow QRS had a similar benefit if the lead was placed in the correct spot. In other words, there is a statistical difference between having the wrong patient with the lead in the right spot and the right patient with the lead in the wrong spot. It can therefore be concluded that patient selection is important for successful outcomes in CRT, but it is only one component of a complex picture.
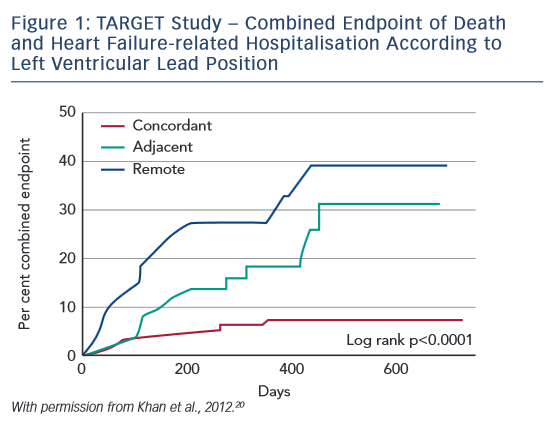
In the Targeted Left Ventricular Lead Placement to Guide Cardiac Resynchronization Therapy (TARGET) study, patients (n=220) underwent baseline echocardiographic speckle-tracking two-dimensional radial strain imaging and were then randomised 1:1 into two groups. In group 1 (TARGET), the LV lead was positioned at the latest site of peak contraction that was free from scar and non-apical. Group 2 (control) patients underwent standard unguided CRT. Patients were classified by the relationship of the LV lead to the optimal site as concordant (at optimal site), adjacent (within 1 segment), or remote (≥2 segments away). The primary endpoint was a 15 % reduction in LV end-systolic volume at 6 months. Secondary endpoints were clinical response (one improvement in NYHA functional class), all-cause mortality and combined all-cause mortality and HF-related hospitalisation. Response rates were higher in TARGET patients (83 % versus 65 %; p=0.003), and superior improvements in ESV and QOL were also reported.20 The major finding of this study was the importance of lead placement exactly at the latest zone, not merely adjacent to the latest zone (see Figure 1). These study data can be summarised in Concept 3: electrical mapping is effective and improves outcomes for patients undergoing CRT.
Dr O’Donnell proceeded to describe how electrical mapping is used in his institution: following lead placement, activation is measured from proximal to distal, which shows an activation wave front. By using the electrical map to achieve a more distal lead placement, improvements can be gained. In the ongoing ‘moving the LV lead quad lead’ study, in patients where the lead was moved, QLV at final position was 89 % whereas in patients where the lead was not moved, QLV in final position was 77 %,21 leading to Concept 3b; electrical mapping is effective and improves outcomes for patients undergoing CRT. However, electrical mapping requires moving the lead if it is not in the late zone.
There is a reluctance among cardiologists to move leads and, in these situations, a quadripolar lead is useful. A recent multicentre study of consecutive implants and a quadripolar lead has shown that the mean difference in intrinsic electrical activation of the usable electrodes was 30 ms (3 to 55 ms). Quadripolar leads reduce phrenic nerve stimulation, reduce high thresholds and are user friendly; however, the most compelling argument for their use is the fact that resynchronisation parameters can be improved by 20 ms by picking the best versus worst electrode.
The optimal pacing site appears to be determined by the fastest pathway of activation from the LV and RV electrodes.22 If it takes longer to move from one side of the heart than from another, the answer is to move the leads. The greater the difference in timings left–right versus right–left, the worse the outcomes, i.e., delta LV. In a recent study, the RV-paced delay (RVp-LV) and LV-paced delay (LVp-RV) were measured during RV-only pacing and LV-only pacing, respectively. The timing difference between the LVp-RV and RVp-LV was termed the delta LV. Results showed that significant intrinsic electrical delay and shorter delta-LV both predicted response, even when LV leads were implanted in the targeted mechanically delayed segment. Such assessments of electrical dyssynchrony may be used to determine optimal lead positions and response to CRT.23
In conclusion, electrical mapping is very effective but is not yet easy. Anatomical LV lead placement does not enable optimal patient outcomes but electrical mapping can guide LV placement and improve outcomes for patients undergoing CRT. Increased experience will improve our expertise at mapping CRT. However, by using the lead with the most poles and the device with the most programming options, we can future proof our patients.








