AF is the most common sustained cardiac arrhythmia in clinical practice. It is associated with increased risk of stroke and heart failure (HF), and is a significant global health challenge.1 Catheter ablation procedures, which isolate the pulmonary veins (PV) from the left atrium and prevent AF initiation, are effective and safe treatment options, and have emerged as the preferred rhythm control strategy for symptomatic paroxysmal AF refractory or intolerant to at least one antiarrhythmic medication.2,3 This procedure is associated with a high success rate (>70%) in paroxysmal AF, but with persistent AF, the success rate after a stand-alone pulmonary vein isolation (PVI) approach remains lower (about 50–60%).1
Due to worse long-term outcomes in patients with persistent AF, additional substrate ablation is frequently performed.4 Scar tissue is present in many of these patients, especially those with persistent AF, but is also seen in those with paroxysmal AF. This scarring is associated with poor outcomes after PVI.5–7 This has led to the development of ablation strategies to eliminate low voltage regions caused by scarring in the left atrium. This article aims to discuss the challenges of atrial scarring in patients with persistent AF and substrate mapping strategies, which allow improved arrhythmia freedom rates after catheter ablation therapy targeting arrhythmogenic atrial substrate.
The challenges of atrial scarring
Understanding the AF substrate is essential to improving outcomes in catheter ablation of patients with persistent AF, and it can enable individualised treatment approaches.8–10 A growing body of evidence indicates that pre-existing or iatrogenic atrial fibrosis, or scarring, plays a role in the maintenance of AF.10,11 Animal studies have shown that propagation of fibrillation waves is promoted by endocardial bundles in acute AF and by epicardial bundles in persistent AF.12 Atrial fibre bundle rearrangement results in more perpendicular orientation of epicardial to endocardial bundles, causing endo-to-epicardial dissociation of electrical activity and the formation of a 3D AF substrate.12 However, while the definition of ventricular scar is well established, this approach has been more challenging in the atrium, due to the structure of the atrial wall and the difficulty of detecting atrial fibrosis with existing imaging techniques.13
Atrial fibrosis can separate cardiomyocyte bundles, which can hinder electrical coupling and slow electrical conduction.14 The presence of left atrial (LA) scarring in AF patients has therefore been associated with an abnormal electroanatomic substrate causing lower regional voltage, increased proportion of low voltage, slowed conduction and increased proportion of complex signals compared with controls.7 In a study of 700 consecutive patients undergoing PVI for the first time, pre-existing LA scarring was a strong independent predictor of procedural failure and was associated with a lower ejection fraction (EF), larger LA size, and increased inflammatory markers, with patients experiencing left atrial scarring having a significantly higher AF recurrence (57%) compared with patients without scarring (19%, p=0.003).5 In a multicentre, prospective, observational cohort study of patients diagnosed with paroxysmal and persistent AF undergoing their first catheter ablation with PVI, atrial tissue fibrosis estimated by delayed enhancement MRI (n=272) was independently associated with the risk of recurrent arrhythmia.6
A promising new strategy for ablation of AF is atrial scar-based catheter ablation to modify low voltage regions that may indicate scar and/or zones of non-uniform anisotropic conduction in the left atrium and convert them into electrically silent regions.9,15,16 In a study that compared the two-year outcomes in patients with paroxysmal AF and severe LA scarring identified by 3D mapping, undergoing PVI-only or PVI plus scar homogenisation, the latter group had significantly better long-term outcomes than those who had PVI alone. While single procedures had a low success rate, ablation of non-PV triggers during repeat procedures resulted in significantly better outcomes.17
Methods of Analysing the Extent and Severity of Scarring Pre-procedure
A number of studies have concluded that substrate mapping is a useful tool to guide personalised AF substrate modification in patients undergoing AF ablation (Table 1).9,10,18–20 In a study that compared electrophysiologic abnormalities in 80 patients with AF (30 paroxysmal AF, 22 persistent AF and 28 long-standing AF), with 20 matched controls, high-density 3D electroanatomic mapping showed that the low voltage index increased gradually from control to paroxysmal AF, persistent AF and long-standing AF.21 In a single-centre randomised study, individually tailored substrate modification guided by voltage mapping was associated with a significantly higher arrhythmia-free survival rate compared with a conventional approach applying linear ablation according to AF type.22 A recent meta-analysis concluded that voltage-guided substrate modification by targeting low-voltage areas in addition to PVI is more effective and safer than conventional ablation approaches, though cautioned that more randomised studies are needed.23
The 2017 Heart Rhythm Society expert consensus document assigned a class IIb recommendation to mapping and ablation of areas of abnormal myocardial tissue identified with voltage mapping as an initial or repeat ablation strategy for persistent AF. A class IIb recommendation was also given to creation of linear ablation lines (in the absence of documented macro-reentrant flutter), ablation of complex fractionated atrial electrograms, rotor ablation, extensive posterior wall ablation or targeting of autonomic ganglionic plexi in persistent AF. Ablation of non-PV triggers, if found, was given a class IIa recommendation.24
The most commonly used cardiac mapping approach is isochronal or activation mapping, which is based on non-fluoroscopic visualisation of mapping catheters and a partial model of the wavefront excitation sequence created by the manipulation of a mapping catheter. Mapping systems create a 3D reconstruction of the area of interest and enable the location of catheters, maps of the atria, colour-coded activation and voltage maps and the tagging of regions of interest.25
The most widely available electroanatomical-mapping systems are the CARTO® (Biosense Webster), the EnSite NavX and the EnSitePrecision® system (Abbott).26–28 The CARTO system comprises three active weak magnetic fields (5×10−6 to 5×10−5 Tesla), produced by a three-coil location pad placed underneath the patient’s thorax. Its catheter tips contain a magnetic mini-sensor that continually measures the strength of the magnetic field and calculates the catheter’s exact position.29 The CARTO-3 system features the HD colouring/confidence algorithm, which allows for automated map acquisition. The EnSite Precision system has new features including the EnSite AutoMap Module, which allows the physician to map arrhythmias faster than current systems.28 These systems have been shown to reduce radiation and the duration of procedures.27,30 More recently, the Rhythmia HDx (Boston Scientific) mapping system has been introduced. This uses a combination of magnetic-based tracking for a sensor at the catheter tip and impedance-based tracking for all electrodes, enabling the rapid and automatic acquisition of maps with high resolution and without the need for extensive manual annotation.31–33
Mapping approaches may be challenging. Cardiac mapping can be difficult if scarring is present because of lack of capture in these areas of low voltage.34 The difficulties arise from a poor understanding of events such as collision waves and anisotropy in the low voltage and fragmented areas and how to modify these abnormal electrical areas to eliminate or homogenise them. It can also be difficult to define what is healthy and what is diseased myocardium. The optimal voltage threshold that defines scar areas has yet to be defined for voltage maps created with conventional ablation catheters and for multipolar catheters. The conventional voltage cut-off is set at <0.5 mV for defining scar areas that have been targeted during ablation. Recent studies suggest that other cut-off values could be used in detecting deceased atrial myocardium.35,36 Scar maps obtained by electroanatomic mapping have emerged as a useful tool to guide personalised AF substrate modification in patients undergoing AF ablation, and is supported by a growing body of evidence.16,36 There are currently no data on ablation of atrial fibrosis guided by delayed-enhancement MRI. The ongoing Efficacy of Delayed Enhancement MRI-Guided Ablation versus Conventional Catheter Ablation of Atrial Fibrillation (DECAAFII) (NCT02529319) randomised study aims to recruit 888 participants.
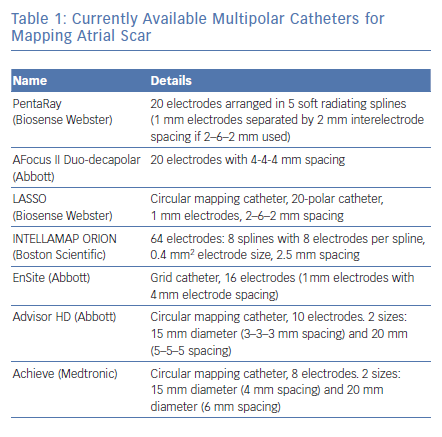
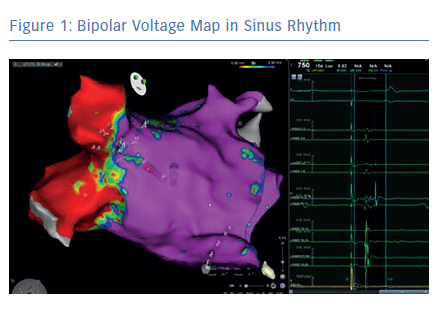
Before the availability of the multipolar mapping catheters, it was much more challenging to map arrythmias in scarred atria. The large tip catheter made it difficult to see any signals in scarred tissue. Large parts of the atria appeared to be completely silent and it was challenging to map the arrhythmia. Localised reentries in scarred tissue were sometimes impossible to see. The use of a multipolar mapping catheter offers an important addition to the voltage map, helping clinicians better understand the arrhythmias (Figures 1 and 2).
The Multipolar Mapping Catheter as a Tool for Substrate Mapping in Clinical Practice
The standard catheter for mapping the left atrium is a linear catheter with a 3.5 mm distal electrode separated by 2 mm from a proximal 2 mm electrode, resulting in a centre-to-centre interelectrode spacing of 4.75 mm.34 In recent years, a number of multi-electrode contact-based catheters have been introduced into clinical practice. Used in conjunction with a 3D mapping system they allow the sampling of multiple points of cardiac mapping data. The devices usually contain more than 20 electrodes. The catheters’ contact with the endocardium surface is atraumatic and provides a high-density map with an interelectrode spacing down to 1 mm.20
Multielectrode catheters were introduced in the 1990s for the diagnosis of supraventricular tachycardia; however, their use for scar mapping did not begin until the 2000s with the introduction of electro-anatomical mapping systems.37 Compared with point-by-point voltage maps, multipolar maps have been shown to have significant advantages, such as higher mapping resolution that can identify heterogeneity in the area of low voltage, localising channels of surviving bundles; the ability to record higher bipolar voltage amplitude with shorter electrogram duration; and pacing with capture at lower output because of increased electrical density.34,38 Electrogram voltages are dependent on the electrode size and spacing, and the angle of the incoming wavefronts to the catheter.
Several multipolar electrode systems are available (Table 1). Circular mapping catheters are the most established. The LASSO circular catheter (Biosense Webster) has 10–20 electrodes and its utility has been demonstrated in a number of studies. A study of 40 maps from 20 patients showed a shorter LA mapping time with greater high definition LA mapping compared with an ablation catheter (13.3 versus 34.4 minutes to reconstruct LA voltage map with 923 versus 228 points).39 More recent systems include the PentaRay® (Biosense Webster) and the AFocus II® Duo-decapolar® (Abbott) catheters. The AFocus II Duo-decapolar catheter has 20 electrodes with 4-4-4 mm spacing and has been used in VT and AF mapping.40,41
The PentaRay catheter has 20 electrodes arranged in five soft radiating splines (1 mm electrodes separated by 2 mm interelectrode spacing) laid out flat to cover an area with a 3.5 cm diameter. It can only be used with the CARTO mapping sytems.26 The multi-branch configuration provides broader access to information with high resolution.
The use of the PentaRay catheter has been demonstrated in a number of case reports and studies of patients with AF.19,20,34,42,43 In a recent prospective study – Substrate Ablation Guided by High Density Mapping in Atrial Fibrillation (SUBSTRATE HD) (NCT02093949), AF was sequentially mapped in both atria of 105 patients admitted for AF ablation, using the PentaRay catheter. Radiofrequency times and procedure times were shorter with the PentaRay catheter compared with a validation set in which a conventional ablation approach was used.19
The IntellaMap Orion™ (Boston Scientific) multipolar catheter has 64 electrodes. This system comprises eight splines with eight electrodes per spline, 0.4 mm2 electrode size and 2.5 mm interelectrode spacing and is used in combination with the Rhythmia mapping system.44–47
The EnSite high density grid catheter has 16 electrodes (1 mm electrodes with 4 mm electrode spacings), and is designed for use with the Precision platform. The four-spline design reduces variability of maps associated with differences in orientation of the catheter relative to the propagating wavefront. Early clinical data suggests that this system enables the rapid collection of a high density of voltage points.48
Other catheters include the Advisor HD™ (Abbott), which is a 20-pole circular mapping catheter, and the Achieve™ advanced mapping catheter (Medtronic).49 These catheters have a low bipolar electrical resolution because of long interelectrode distances.50 Each of these catheters have their own unique characteristics and it is important to individualise scar thresholds.
Conclusion
Catheter ablation for the treatment of persistent AF is associated with suboptimal outcomes, largely due to the presence of scar tissue. AF recurrence is frequently reported, and patients often undergo repeat ablations after conventional PVI procedures. Substrate-based modification via targeting areas of scar in the left atrium has therefore emerged as a promising therapeutic strategy. Multipolar catheters with smaller electrodes and shorter interelectrode distances are more effective tools for high-density mapping than conventional catheters. Their use has facilitated the visualisation of areas of low-voltage or scar and has allowed the operator to effectively modulate or eliminate arrhythmogenic substrate and improve the prognosis of patients with highly symptomatic AF.
In order to optimise accuracy and reproducibility of scar maps, there is a need for standardised protocols and uniform cut-offs for scar detection. While large randomised controlled trials are needed to confirm the benefits, clinical evidence to date suggests that the use of the multipolar mapping catheters may facilitate successful ablation of scar-related AF.
Clinical Perspective
- Pulmonary vein isolation is the cornerstone of catheter ablation of atrial AF.
- Atrial fibrosis has been demonstrated to predict likelihood of success after AF ablation. A new strategy for ablation of AF is substrate-based ablation of fibrotic areas visualised as low-voltage areas on electroanatomical maps. The PentaRay® catheter (Biosense Webster) allows for fast mapping with high density and high resolution facilitating the creation of voltage maps.
- The use of large-tip catheters caused difficulties and occasionally the inability to detect signals in scarred tissue. Localised reentries in scarred tissue were sometimes impossible to see.
- Multipolar catheters with closer spacing and smaller electrodes have higher resolution which allows the detection of signals in previously ‘silent’ areas.