Right ventricular apical pacing (RVAP) results in dyssynchronous ventricular activation that can lead to impairment of ventricular function. Alternative myocardial pacing sites such as RV septal pacing (RVSP) and RV outflow tract pacing still rely on myocardial cell-to-cell conduction and have not been shown to prevent pacing-induced cardiomyopathy.1 Biventricular pacing (BVP) certainly improves upon RVAP, but still produces a non-physiological activation pattern.2 Direct pacing of the His–Purkinje conduction system offers the ability to preserve physiological activation of the ventricles in patients with intrinsically normal, narrow QRS complexes. In patients with bundle branch block (BBB), conduction system pacing can deliver cardiac resynchronisation therapy (CRT) by correcting BBB to synchronise ventricular activation.3
The originally favoured site of conduction system stimulation is the His bundle, and there is now large global experience of pacing at this site with considerable published follow-up data. More recently, new techniques have pulled focus to pacing of the region of the left bundle branch, which has a growing evidence base.4 In this state-of-the-art review of His–Purkinje conduction system pacing, we assess recent evidence and current practice and explore emerging and future directions in this rapidly evolving field.
Terminology
His Bundle Pacing Terminology
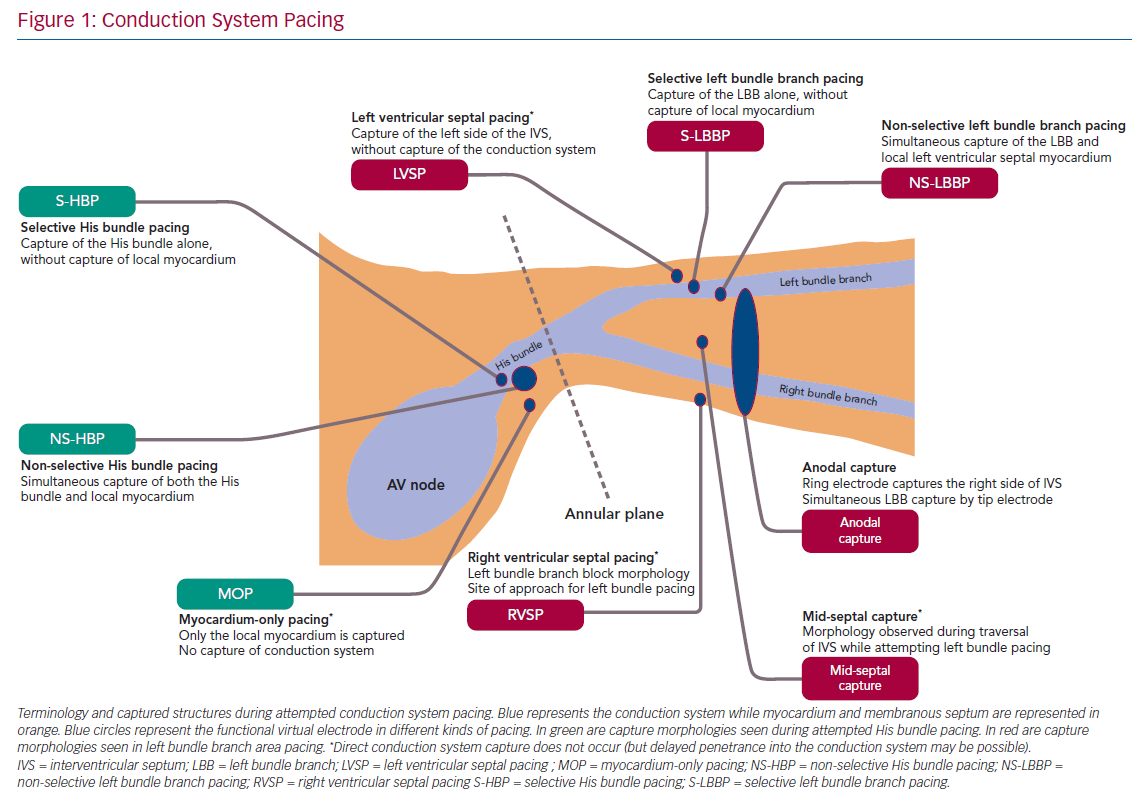
The classification and nomenclature of conduction system pacing has changed since its early period,5 and many definitions are now standardised.6,7 Early discussion of His bundle pacing (HBP) made reference to direct HBP8 as well as to para-Hisian pacing.9 Selective and non-selective HBP (S- and NS-HBP, respectively) are now the two terms used for capture of the His bundle, and their features are outlined in this review. S-HBP results in capture of the His bundle alone without myocardial capture. In NS-HBP, in addition to HBP there is capture of surrounding septal myocardium, resulting in septal pre-excitation for most of the duration of His–ventricular (HV) conduction time.
Bundle Branch Block Terminology
When HBP is able to narrow the QRS of patients with left or right BBB, varying terms are used to evoke varying explanations of underlying phenomena. ‘His resynchronisation’ or ‘His-CRT’ do not specify a mechanism of QRS shortening.2 ‘Bundle recruitment’ refers to capture of the previously non-functional conduction fibres and the term is used to differentiate this from fusion of myocardial wavefronts, which can produce QRS narrowing when NS-HBP fails to recruit the right bundle in patients with right bundle branch block (RBBB).
Left Septal Pacing Terminology
Understandably, given its relatively recent emergence, there is considerably more variation in naming convention in contemporary discussion of pacing the left bundle branch and the surrounding region. Left bundle branch pacing (LBBP) more precisely describes the relevant region of conduction system than ‘left bundle pacing’ given that the longitudinally dissociated and continuous left bundle fibres (both before and after branching) originate in the His bundle. ‘Left bundle branch area’, ‘peri-left-bundle-branch’ and ‘deep septal’ pacing/capture are also generally used interchangeably to refer to the general trans-interventricular septum (IVS) approach to attempt LBBP but do not specify if conduction system capture is achieved. Selective and non-selective LBB capture are classified similarly as with HBP but, due to the position of the lead ring, anodal capture of the right side of the IVS can also be seen with bipolar deep septal pacing. With emerging evidence that endocardial left ventricular (LV) pacing may involve deferred penetrance of activation wavefronts into the conduction system, the term ‘direct’ may return with LBBP to differentiate direct capture of conduction system from this indirect phenomenon.10,11 Figure 1 illustrates the current landscape of terminology, anatomy and conceptual classification of conduction system pacing.
Potential Indications for Conduction System Pacing
There are three broad categories of potential indication for conduction system pacing: when a high burden of ventricular pacing is necessary, which includes atrioventricular block (AVB), slowly conducted AF, pacing-induced cardiomyopathy and atrioventricular nodal ablation (AVNA); CRT in patients with heart failure and BBB; and sinus node dysfunction (SND), where AV nodal conduction disease may already coexist or develop during follow-up, and operators can gain experience in conduction system pacing because implant failure is less problematic. Given that HBP has been performed more widely, large registries have collated international experience to provide a picture of contemporary practice, including indications.6,12,13 In the Keene et al. multicentre registry of 529 patients, AVB was the most common indication, seen in half of cases, with slow AF the next most common (27.8%).13 The remainder of the patients had CRT, SND and AVNA in similar proportions (6.6–8.9%). In the Zanon et al. 844 patient multicentre experience, AVB (41.2%) and AF (39.7%) were also the most common indications, but fewer patients underwent His-CRT (1.7%).12 First-degree AVB with narrow QRS, where conduction system pacing can be used to shorten AV delay while preserving physiological ventricular activation, stands apart as a potential indication, and HBP for this indication is being tested in the His Optimized Pacing Evaluated for Heart Failure (HOPE‐HF) blinded randomised cross-over trial.14 The early experience of LBBP indicates a similar range of indications but with small numbers of patients in published series, the relative proportions are difficult to ascertain. In principle, there is no reason for indications to differ between HBP and LBBP, with the exception of CRT for RBBB where LBBP needs to be carefully studied.
Conduction System Pacing Techniques
History of the HBP Technique
Stimulation in the region of the cardiac conduction system to achieve physiological ventricular activation and normalised QRS appearance through direct capture of the His bundle or bundle branches was first reported in humans in 1970.15 Temporary para-Hisian pacing has been a standard manoeuvre in electrophysiological (EP) studies for decades,16 but the implantation of an actively fixed His bundle lead was first described in 2000.8 The initial technique involved mapping the region of the His bundle using a steerable catheter inserted via the femoral vein prior to a carefully shaped stylet being used to guide a lead to the mapped His bundle.17 This cumbersome method was refined to the modern stylet-less technique, where a lumenless lead is steered towards the His bundle, a right atrial structure found at the inferior interatrial septum immediately superior to the tricuspid valve, using a pre-shaped sheath or a deflectable sheath.16 Although in its infancy this technique was supported by EP catheter mapping of the region, the ability to map signals from the conduction system and adjacent myocardium and using the lead within the sheath was subsequently described by the Geisinger HBP group.18
Modern HBP Technique
The His signal and appropriately balanced atrial and ventricular components (typically a ventricular signal at least twice the amplitude of the atrial signal) are identified on the lead electrogram (EGM). EGM and ECG characteristics (Table 1) during pacing can confirm the suitability of the location for fixation. The lead is slowly manually rotated through fewer than 10 complete revolutions (typically five). The modern technique utilises the property of the SelectSecure 3830 lead (Medtronic), where the exposed helix is a constituent part of the tip electrode, rather than only the lead tip itself, proximal to the screw, so that when the screw penetrates the fibrous capsule of the His bundle, the conduction system fibres within the His bundle can be captured at relatively low thresholds.
Several registries have demonstrated that the stylet-less technique using a SelectSecure 3830 lead is effective in achieving HBP.6,12,13 In the vast majority of cases, the C315 fixed curve workhorse sheath (Medtronic) is sufficient to reach the His bundle, but in a sizeable minority the deflectable C304 deflectable delivery sheath (Medtronic) is used, with a yet smaller minority requiring modifications to coronary sinus sheaths.19 The C315 has a primary curve to direct leads anteriorly towards the tricuspid annulus and a secondary curve to reach the septum.
Left Bundle Branch Pacing Technique
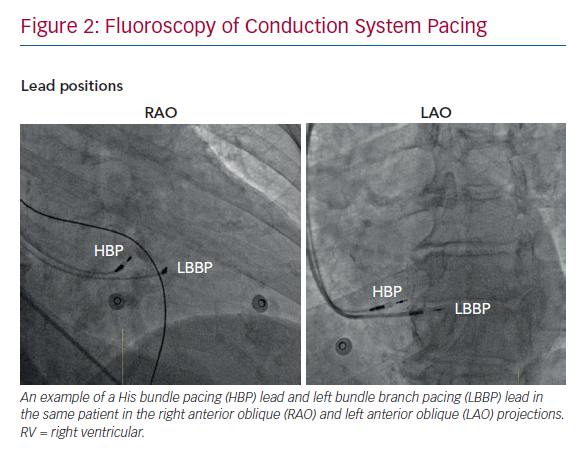
The technique for directly pacing the left bundle branch was first reported by Huang et al. in 2017.20 The 3830 lead was deployed deep in the IVS, 15 mm distal to the His bundle site in a patient whose LBBB was not corrected by HBP. Pacing at this site successfully narrowed the QRS duration with a response consistent with conduction system capture. The technique for this trans-IVS approach to LBBP is now more firmly established.21 The HBP technique is used to first identify the distal His bundle, before moving the sheath tip 1–2 cm more distally along the RV septal surface toward the RV apex (Figure 2). Fixating a lead into the His bundle as an anatomical landmark is useful in challenging cases. Pacing at the distal site will produce an LBBB-type pattern including a negative QRS with W-shaped notching in lead V1. Rather than the small number of slow turns recommended for HBP, LBBP requires several bursts of multiple rapid rotations of the SelectSecure 3830 to progress the lead 6–8 mm through the IVS. This may result in a total number of revolutions several times higher than performed in HBP. Periodic checking of the paced QRS characteristics (Table 1) and of lead impedance should be performed after each burst of rotations to confirm if LBB capture has been achieved and to ensure that the lead does not perforate through to the LV cavity, respectively. Contrast injection through the sheath, measuring the point of the lead fulcrum (at the cavity–septum interface) and echocardiography (transoesophageal, intracardiac and, sometimes, transthoracic) can help to identify the depth of lead penetration through the IVS.
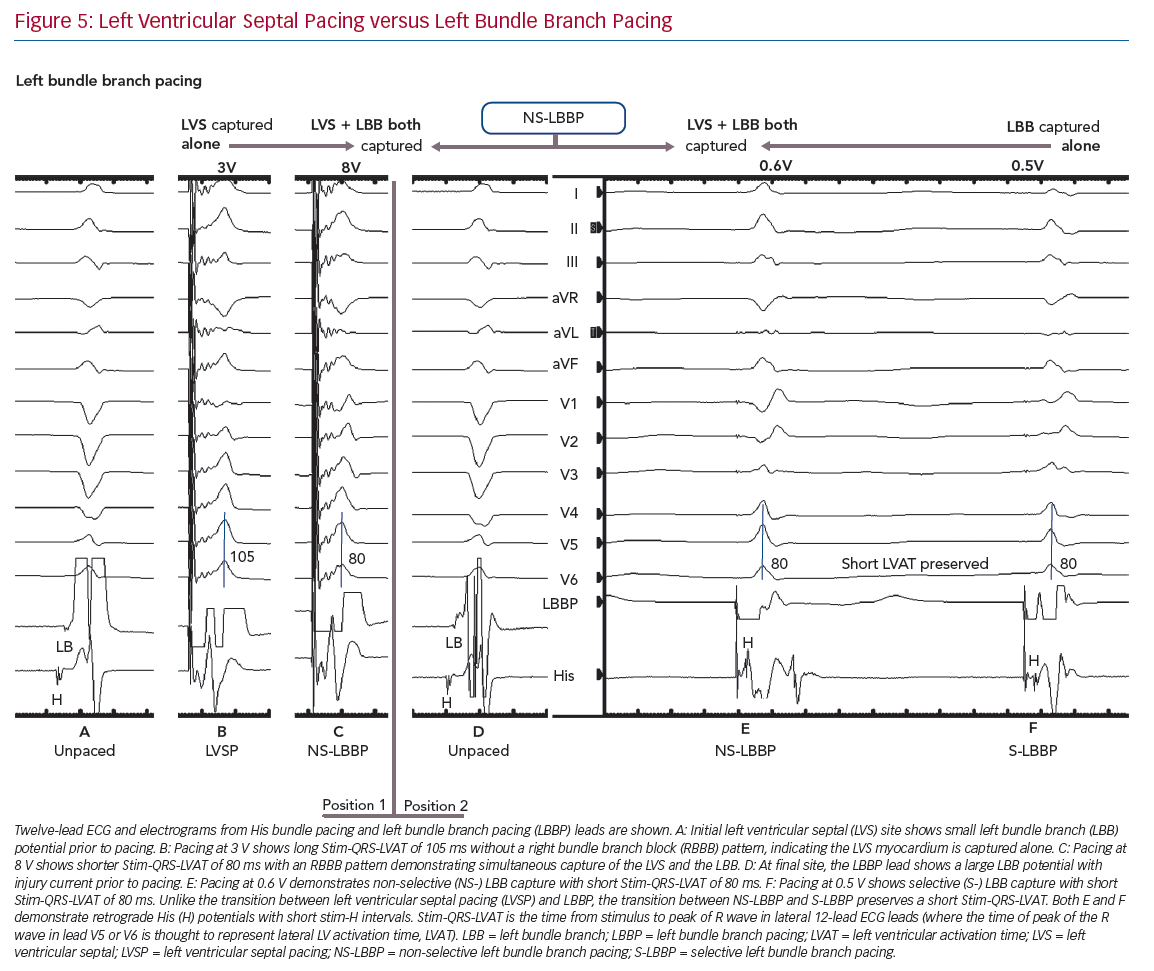
The paced QRS will change in morphology as the lead progresses through the mid-septum to the left side of the IVS. In lead V1, the emergence of an RBBB type pattern with a notch/R’ wave, thought to represent RV activation, moves later in the QRS complex, the deeper into the septum the lead progresses. The time of peak of the R wave in lead V5 or V6 is thought to represent lateral LV activation time (referred to here as QRS-LVAT to distinguish this measure from LVAT measured using other techniques). The time from the stimulation artefact to QRS-LVAT (Stim-QRS-LVAT) gradually shortens the deeper into the septum the lead is progressed until a step change occurs and Stim-QRS-LVAT substantially shortens to less than 80 ms as left bundle capture is achieved (Figure 3). A left bundle potential may now be seen on lead EGM during intrinsic conduction. In general, the transition from pacing at the RV septum, through the mid-septum to the left septum, where the left bundle can be captured, can be thought of as an LBBB-type paced QRS morphology changing into an RBBB type. There are related techniques that produce deep septal pacing, but do not necessitate conduction system capture, however, it is hypothesised that such techniques, along with trans-interatrial septum endocardial LV pacing, may involve a degree of direct or delayed conduction system capture.10,11,22
Pacing Characteristics in Conduction System Pacing
Capture Characteristics in His Bundle Pacing
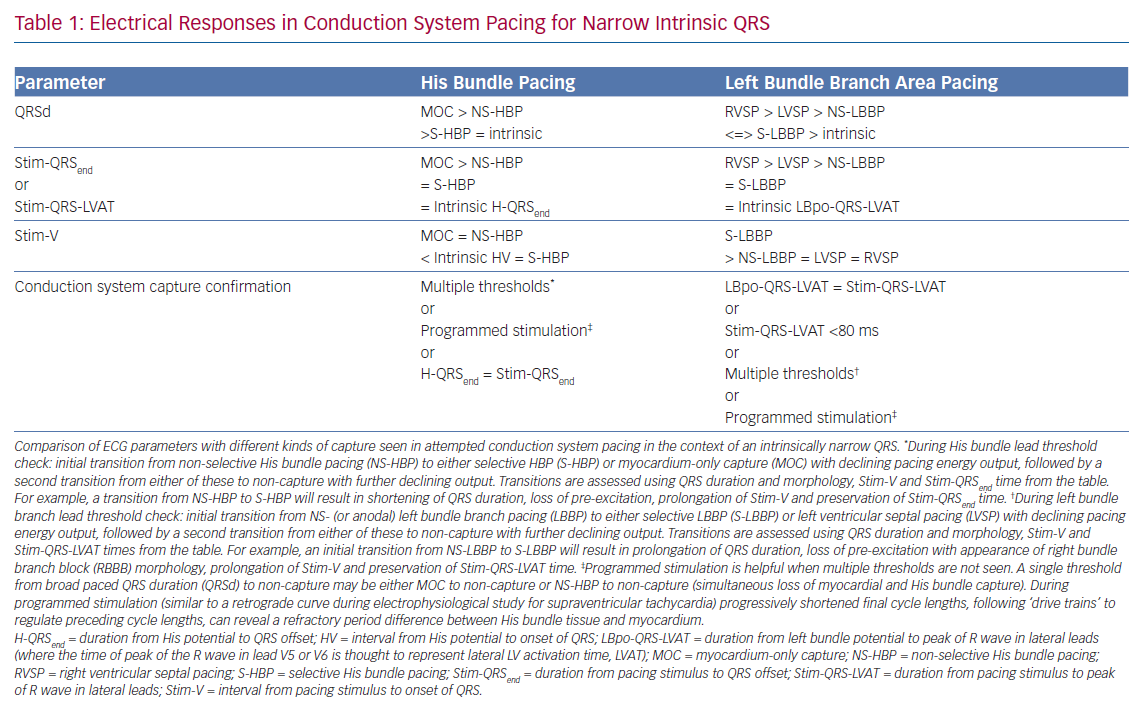
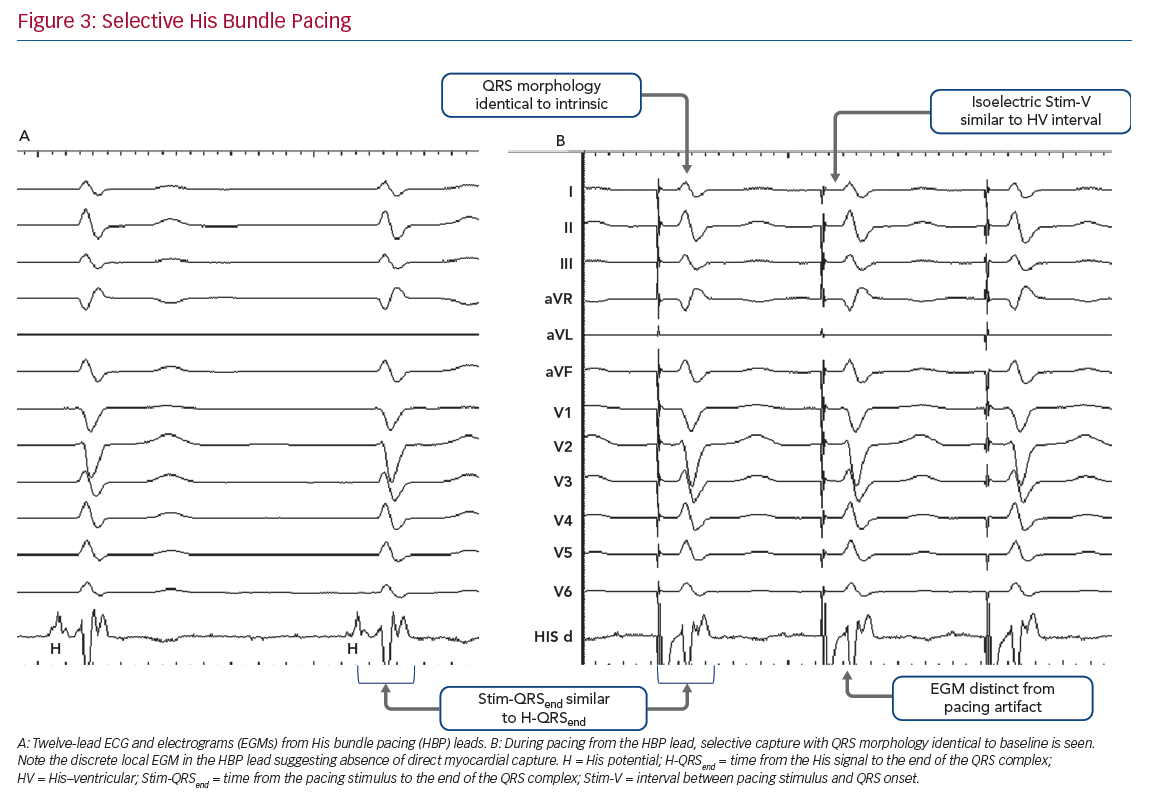
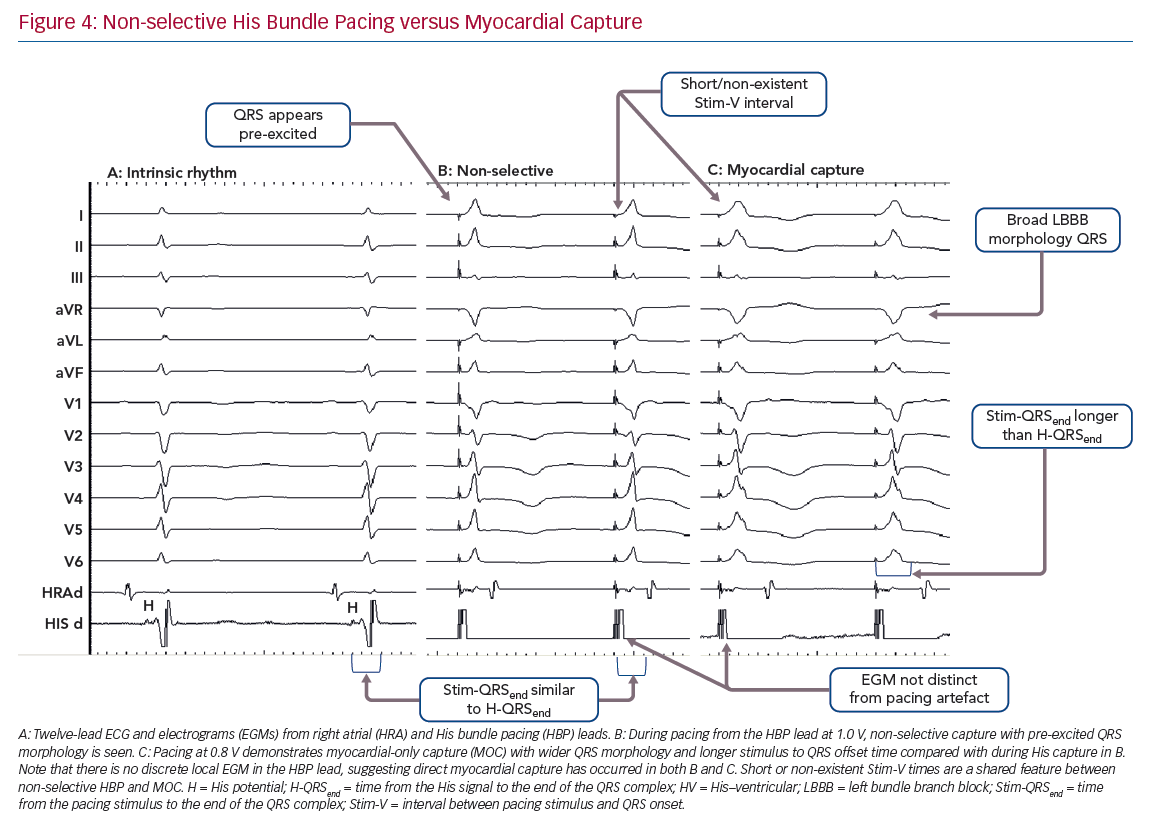
Selective HBP occurs when the His bundle is captured without capture of surrounding local myocardium. In patients with a narrow intrinsic QRS complex, this manifests on 12-lead ECG as an iso-electric interval between the pacing stimulus and the QRS onset (Stim-V interval) that is usually approximately equal to the unpaced, intrinsic interval from the His signal on the lead EGM to the onset of QRS (HV interval). The paced QRS duration (QRSd) is equal to intrinsic QRSd, because the LV and RV are activated entirely via the His–Purkinje conduction system, and therefore the time from the pacing stimulus to the end of the QRS complex (Stim-QRSend) is equal to the time from the His signal to the end of the QRS complex (H-QRSend). The local EGM will be discrete from the pacing artefact, suggesting lack of local myocardial capture (Figure 3).
Non-selective HBP occurs when the local septal myocardium is captured alongside capture of the His bundle. During the time where the signal is travelling through the insulated His bundle, local myocardial activation is occurring due to myocardial capture by the pacing stimulus. Therefore, the QRS complex onset occurs very soon, often immediately, after the pacing stimulus, via slow cell-to-cell myocardial conduction through a small region. The remainder of the ventricles are activated rapidly by the His–Purkinje system, therefore ventricular activation (and thus the QRS complex) is completed at an identical duration from the pacing stimulus as in S-HBP, but QRSd is longer in NS-HBP due to early ventricular activation. The slow slurred QRS pre-excitation in NS-HBP is akin to a delta wave in patients with manifest accessory pathways and is referred to as the pseudo-delta-wave. The local EGM is incorporated into the pacing artefact due to local capture (Figure 4).
When the His bundle is not captured but the pacing stimulus nevertheless produces ventricular activation, myocardium-only capture (MOC) occurs. This results in slow cell-to-cell activation of the entirety of both the RV and LV. The measurements that distinguish S-HBP, NS-HBP and MOC are set out in Table 1, showing that H-QRSend is the key reference measurement to distinguish individual NS-HBP complexes from MOC. During non-selective His bundle capture the H-QRSend will be equal to Stim-QRSend. This requires co-visualisation of the lead EGM with the 12-lead QRS, which is easily done on EP laboratory systems. Without the reference H-QRSend interval, a sudden prolongation of QRSd, and transition in morphology, from NS-HBP to S-HBP or MOC with declining pacing output during a threshold check can be seen. This diagnoses NS-HBP and MOC.
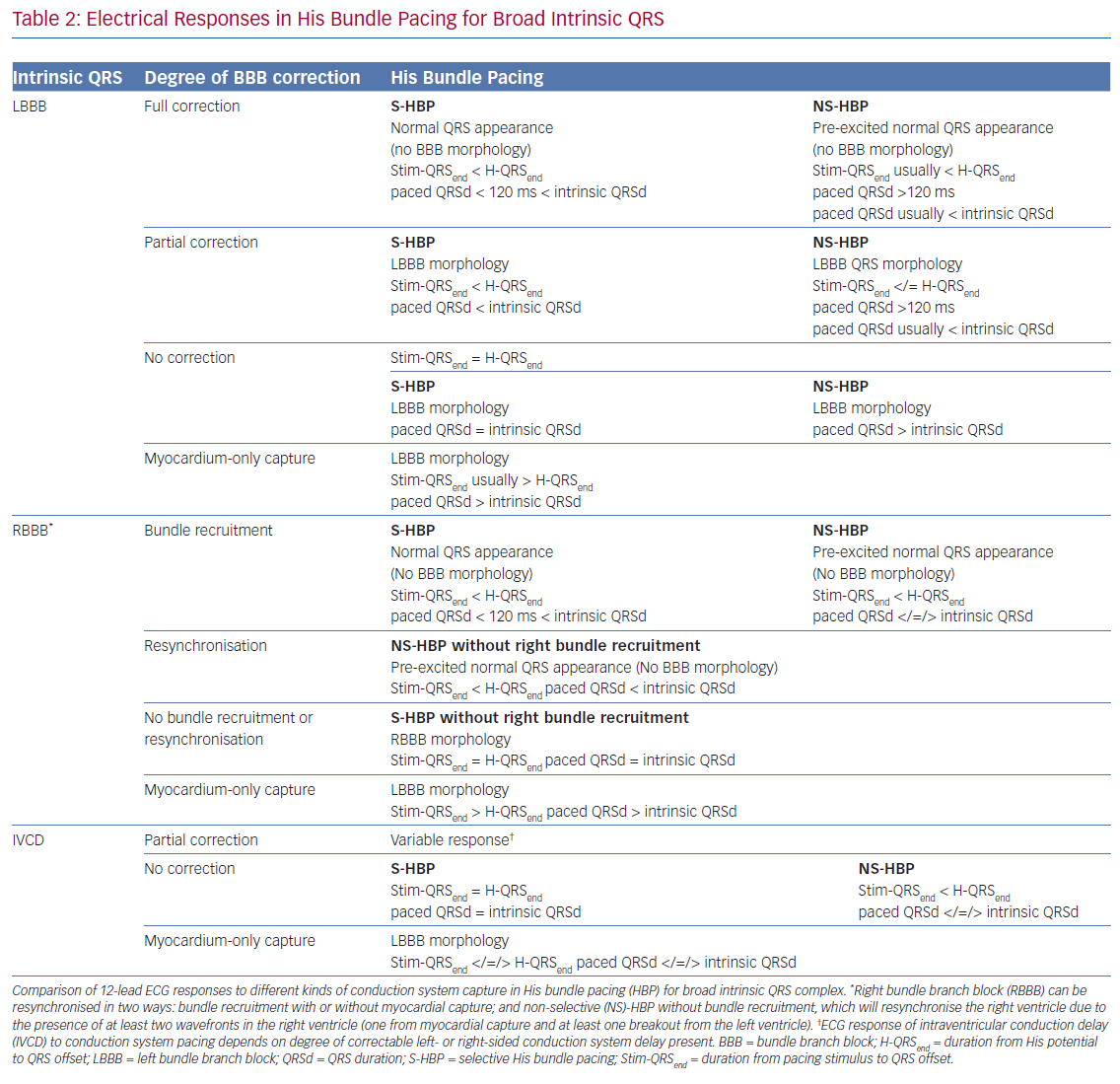
When such a transition is not seen (and a reference H-QRSend measurement is unavailable), there may be either NS-HBP or MOC at all capturing outputs but the distinction cannot easily be made. Recent applications of differences in refractory periods between conduction tissue and myocardium have led to the technique pioneered by Jastrze˛bski et al. to allow HBP-MOC distinction in these cases.23 Programmed stimulation with a fixed S1 drive train and shortening S2 coupling interval can reveal MOC at shortest capturing coupling intervals with NS-HBP at longer coupling intervals. As criteria and manoeuvres for capture confirmation become increasingly complex, the use of artificial intelligence may become important, with proof of concept recently demonstrated.24 Assessing and defining conduction system capture in patients with underlying conduction disease is more complex, and a summary of this is provided in Table 2.
Selective Versus Non-selective His Bundle Pacing
The superiority of NS-HBP over MOC has a clear physiological basis, with only NS-HBP, of the two, involving conduction system capture. Although the QRS appearance of MOC may, in some cases, be only subtly different from NS-HBP, in MOC slow cell-to-cell propagation is responsible for LV activation (rather than rapid and physiological conduction system activation).25 However, the relative merits of S-HBP and NS-HBP are a key ongoing controversy in HBP, with important recent evidence illuminating the issue. The 12-lead ECG appearance of S-HBP suggests that both ventricles are activated physiologically, whereas in NS-HBP there is non-physiological activation of some septal myocardium. The extent to which local myocardial capture is physiologically relevant is of importance, because NS-HBP has some potential advantages compared with selective His capture. First, local myocardial capture allows the potential for continued ventricular pacing in the event of the development of infra-Hisian block. Second, the evoked potential of myocardial capture in NS-HBP can be detected using auto-threshold algorithms, but this does not occur with S-HBP, which limits the value of automatic capture detection algorithms.26
Electrical mapping suggests that local myocardial capture mainly affects the basal-to-mid RV, and mechanical synchrony indices suggest that LV activation is dyssynchronous only when conduction system capture is not present (as occurs in MOC), and that LV dyssynchrony is not induced by NS-HBP.14,27 Measurements using ultra-high-frequency ECG, which can spatially segregate signals within the QRS to measure LV electrical synchrony, corroborate electrocardiographic imaging (ECGI) data that LV synchrony is largely unaffected by NS-HBP in comparison with S-HBP or intrinsic activation.28 Beer et al. compared long-term outcomes of heart failure hospitalisation or mortality between S- and NS-HBP and found no significant difference.29 The region of activation from local myocardial capture is small and similar to accessory pathway pre-excitation, which only rarely causes dyssynchrony-induced cardiomyopathy.30
The current consensus is that in the vast majority of cases, dyssynchrony induced by NS-HBP is minimal, unless intrinsic HV is very long, and mostly isolated to the RV. In relatively rare cases, presumably in those with a genetic susceptibility to dilated cardiomyopathy and/or NS-HBP with considerable myocardial pre-excitation, NS-HBP dyssynchrony may be problematic. This may explain the slight, statistically non-significant, divergence in outcomes between S-HBP and NS-HBP seen in the Beer et al. observational analysis (but this may be due to intrinsic differences in the populations).29
Capture Characteristics in Left Bundle Branch Pacing
LBBP also demonstrates selective and non-selective conduction system capture, and LV septal pacing without direct conduction system capture is the equivalent of MOC. Jastrze˛bski et al. showed that programmed stimulation can also be helpful in LBBP.31 However, capture characteristics of LBBP are more complex than HBP. LBBP will result in an RBBB-type morphology. The second component of the QRS (R’) represents RV activation; thus, QRS offset does not demarcate the end of conduction-system activated myocardium. LBBP QRS durations may therefore be longer than intrinsic QRSd. Therefore Stim-QRS-LVAT measurements, using the peak of the R wave in lateral leads, are preferred. This provides a method for assessing the time to lateral LV activation, which is expected to occur via left conduction system capture. The current convention is to assume that left conduction capture has occurred when Stim-QRS-LVAT is shortened to <80 ms. Left bundle potentials are seen with varying frequency (in contrast to the near ubiquity of His potentials in HBP), and the interval from potential to QRS onset is typically 15–35 ms. NS-LBBP can be differentiated from LVSP (without LBB capture) using Stim-QRS-LVAT, but recent evidence from Salden et al. suggests that the importance of this distinction is not as clear cut as the NS-HBP/MOC distinction.32 LVSP without direct/immediate LB capture has similar electromechanical characteristics to BVP and HBP. This may be due to delayed/indirect penetrance of left-sided conduction system, or due to the balanced position of the LV septum with regard to intra-LV and inter-ventricular synchrony.
In general, during LBBP, lead V1 demonstrates RBBB morphology. To confirm LBB capture, in addition to an RBBB paced pattern one or more of the following criteria should be present: presence of LBB potential; evidence for transition from non-selective LBBP to either selective LBBP or LVSP during threshold testing; short and constant Stim-QRS-LVAT <80 ms at high and low outputs; and direct LBB capture demonstrated by short retrograde His or anterograde distal conduction system potentials or programmed stimulation to demonstrate LBB capture (Figure 5).
Sensing From Conduction System Leads
R wave amplitudes sensed from His leads are generally lower than 5 mV and atrial EGMs of varying amplitudes may also be present.3 Therefore, there is the potential for ventricular under-sensing and possible atrial over-sensing. LBBP leads that are surrounded by abundant myocardium have larger R waves, providing one very clear advantage over HBP.10
Thresholds in Conduction System Pacing
The HBP thresholds are typically higher than for RV myocardial capture, but large contemporary registries suggest that improvements in technique have considerably reduced this issue, with mean His capture thresholds of 1.4 ± 0.9 V at 0.8 ± 0.3 ms, and 1.6 ± 1.0 V at 0.8 ± 0.4 ms observed in the two recent large registries.8,12,14 The Keene et al. registry showed that there is a learning curve with HBP and that after 30–50 cases the implant threshold is reduced, as is fluoroscopy time.14 Recent insights into the importance of His injury currents to determine conduction system lead fixation have also improved thresholds.13
LBBP thresholds have been noted to be very low (usually <1 V at 0.5 ms) since its inception and low thresholds are reported in every series.10,33–35 Post-implant threshold rises are observed in around 7% of cases in HBP and may be due to micro-displacement or fibrosis. They occur frequently enough to encourage some operators to implant back-up RV leads (although this practice is declining).14 They can occur early (prior to initial follow-up) but very late rises have also been seen >6 months or even 1 year after follow up despite stable, low intervening thresholds.12 Such threshold rises have not been seen yet with LBBP, which is promising, but this is in the context of a much smaller published experience and short-term follow-up.
Outcomes in Conduction System Pacing
Success Rates and Safety Profile of Conduction System Pacing
Reports of HBP implant success rates range from 72 to 92%, but success definitions have not always been standardised and lower rates are seen with His-CRT.3,13,36,37 Transient AVB and RBBB can be seen during implant. Macro-displacements are rare but rising thresholds are not uncommon. Combining macro-displacement and high threshold as indications for redo procedures, the re-intervention rate is between 6% and 8% in larger long-term studies.12,13,37,38 The early indications are that LBBP has a high success rate (>80%) with low re-intervention rates, and that lead perforation of the deeply fixated LBB lead into the LV cavity is very rare.39 There may, however, be patient populations for whom LBBP is more challenging, such as patients with extensive septal fibrosis or scarring. Longer term follow-up data from large registries are also awaited.
Clinical Outcomes in Conduction System Pacing
Despite more than 20 years of progressively increasing experience of permanent HBP, several years of widespread global interest and uptake and a sizeable social media presence,40 there have been no long-term, large-scale, clinical outcome driven, randomised controlled trials (RCT) of conduction system pacing. The HOPE-HF trial is due to report in 2020 and is the only large-scale RCT imminent.14 In the first decade of BVP, more than 6,000 patients were randomised to BVP versus standard-of-care trials,but if current trends continue it is unlikely even a tenth of that population will be randomised in conduction system pacing RCTs.39 Indeed the established presence of BVP is a key factor that makes trial design for conduction system pacing difficult, alongside disruption by the novel LBBP technique.39 Therefore, we must rely on observational data to make any inferences about long-term clinical outcomes in conduction system pacing. Improvements in quality of life, 6-minute walk test, LV ejection fraction (LVEF), LV size, heart failure hospitalisations and mortality have been seen with HBP in comparison with RVP. Some of the most compelling evidence comes from a comparison of a hospital performing HBP with a nearby hospital with operators who did not perform HBP, but with otherwise similar populations and standards of care.41 HBP was associated with a statistically significant 29% reduction in the primary outcome of death, heart failure or upgrade to BVP at 2-year follow-up in that 756 patient study, and the effect was most pronounced in the subgroup with >20% ventricular pacing burden, with the 25% event rate for the primary outcome demonstrating that the difference was clinically meaningful in absolute terms.41 Small-scale observational studies of LBBP suggest similar clinical and echocardiographic outcomes, but larger, long-term studies and head-to-head comparisons with RVP, BVP and HBP will be required to fully assess LBBP outcomes.11
Conduction System CRT
The role of conduction system pacing to resynchronise BBB in patients with heart failure is a particular indication for which recent insights have greatly altered our understanding. El-Sherif et al. observed in the 1970s that pacing the distal portion of the His bundle could correct LBBB to create a narrow QRS complex.42 Lustgarten et al. demonstrated that this could be achieved with permanent HBP in 2010.43 Subsequent observational studies show that HBP can shorten QRS duration and improve cardiac function and symptoms in patients with heart failure and LBBB.44–46
Given these data, His-CRT has gained prominence as a bail-out in cases of failed BVP, but the burning question in this field was whether the physiological nature of resynchronisation by His-CRT produced better outcomes than BVP. In 2019, a pilot head-to-head comparison between the two modalities was published – His Bundle Pacing versus Coronary Sinus Pacing for CRT (HIS-SYNC).47 His-CRT produced greater QRSd reduction than BVP but a statistically significant difference in LVEF improvement was not found. Unfortunately, the study suffered from a very high rate of cross-over from the HBP arm to the BVP arm, and the reasons for this illustrate the current challenges facing His-CRT. Half of crossovers were attributed to ECGs showing intraventricular conduction delay rather than LBBB. Thirty per cent crossed over due to inability to correct LBBB.47 Arnold et al. have demonstrated, in a within-patient comparison, that when HBP successfully corrects LBBB, the haemodynamic and electrical improvements are greater than with BVP.2 HIS-SYNC showed that successful His-CRT requires selection of patients with conduction system disease amenable to correction by HBP and that improved implantation tools are required to facilitate correction in these patients.47
Upadhyay et al. have shown the physiological basis for patient selection.48 They found, by studying the left-sided conduction system, that patients with 12-lead ECG appearances of LBBB have variation in the nature of conduction disease. The majority had conduction block within the bundle of His, clearly amenable to correction by HBP. A smaller proportion had proximal conduction block within the proximal conduction system but distal to the His bundle: the block was located in the left bundle branch.48 Such patients may be amenable to HBP correction, but LBBP offers a more plausible corrective method. Importantly, in a sizeable minority of their population of patients attending for ventricular tachycardia ablation (36%), the left-sided conduction system appeared to be intact; QRS widening in these patients was presumed to be due to intramyocardial conduction delay. The 12-lead ECG features of typical LBBB do not seem to reliably discriminate between these groups. Practical methods to distinguish these LBBB phenotypes are required alongside tools dedicated to maximising resynchronisation achieved by conduction system pacing. It should be noted that even though conduction system pacing will not correct it, LV septal pacing may have a role in patients with intra-ventricular conduction delay with intact conduction system. This group includes, for example, a combination of LV hypertrophy and left axis deviation, which can appear on 12-lead ECG as LBBB. LV septal pacing can produce improvements in AV delay in such patients, while activation pattern may be improved compared to the intrinsic pattern.49
LBBP is also able to resynchronise LBBB but the literature is sparse. Published series and case reports include few patients with LBBB.35,49,51 LBBP is promising for CRT due to its presumed ability to correct block within the His bundle and the proximal left bundle. Furthermore, even if the conduction system is not captured, pacing in the LV septum appears to produce similar electromechanical improvements to BVP.32 This potentially makes patient selection less of a problem: even intraventricular conduction delay with intact conduction system (including e.g. LV hypertrophy ECG appearances) might be potentially resynchronised to some degree, and furthermore there is scope for AV delay improvement.50
Conversely, given that LBBP produces an RBBB pattern, HBP is likely to have an advantage over LBBP for resynchronising RBBB. HBP can resynchronise RBBB in two ways: direct recruitment of the right bundle; and NS-HBP results in a wavefront from the basal RV (local myocardial capture) meeting another wavefront originating more apically (from left bundle mediated activation of the RV).52 The HOPE-HF study is recruiting patients with long PR intervals and both narrow QRS and RBBB, and will provide evidence in this group.14
Recent Advances and Future Directions in Conduction System Pacing
New, dedicated HBP sheaths from different manufacturers are on the horizon and it is likely that dedicated equipment for LBBP will follow. Recently some operators have returned to stylet-driven HBP.53,54 This permits variation in lead model as well as an alternate approach in challenging cases. 3D electro-anatomical mapping for pacing the His bundle and left bundle is another area of interest, offering the ability to eliminate or minimise fluoroscopy for the benefit of operators and patients, but restricting practice to operators familiar with mapping and in some cases prolonging overall procedure time.55,56 Alternately, an EGM-only guided approach to successful HBP with minimal fluoroscopy was recently reported by Zanon et al. to be compatible with use of the SelectSecure 3830 lead.57 Automated analysis of HBP ECGs is in development and this has the potential to facilitate even more rapid uptake of the technique.24 Meanwhile, new evidence is gathering regarding the relative efficacy of LBBP compared with HBP, and its ECG characteristics are being more rigorously codified.
Conclusion
Conduction system pacing previously referred only to HBP but is now seen as a collection of techniques: pacing the His bundle, the proximal left conduction system, and the region surrounding it. Initial studies of HBP were mainly confined to single-centre observational series, but widespread interest and uptake of HBP have led to large multicentre, international registries, longer-term follow-up studies and the first RCTs. With more evidence we have gained new insights into the mechanisms of HBP and the nature and magnitude of its benefits, including its ability to prevent pacing-induced cardiomyopathy and to physiologically resynchronise LBBB. Greater scrutiny has also elucidated the limitations of HBP, such as high thresholds, small R waves, long fluoroscopy times and higher failure rates, but larger datasets have also shown that these limitations can be considerably mitigated by operator experience. LBBP has emerged more recently with an impressive rate of accumulation of early evidence. Although it has the potential to address many of the challenges of HBP, its growing evidence base is still sparse and the technique is evolving. Development of newer leads and delivery systems specifically geared towards conduction system pacing addressing the current limitations is necessary to democratise its use. As permanent conduction system pacing enters its third decade, global enthusiasm continues to accelerate and the coming years will hopefully see physiological pacing realise its full potential.
Clinical Perspective
- His bundle pacing has rapidly evolved and has been shown to restore physiologic activation of the ventricles and maintain ventricular synchrony.
- More stable and distal conduction system pacing in the left bundle branch region is a newcomer to the field of physiologic pacing and early evidence suggests it shows promise.
- Randomised controlled clinical trials of the new forms of pacing for bradycardia and resynchronisation therapy are lacking and are essential to gain additional evidence related to the risks and benefit of this approach.