Catheter ablation (CA) is a widely used first-line treatment for AF. Randomised controlled trials (RCTs) have shown its superiority to pharmacological treatment in terms of symptom control, rhythm control and mortality in selected patients; observational studies have also suggested a decreased risk of stroke.1,2 Pulmonary vein isolation (PVI) is the standard endpoint, but studies have reported variable and improvable efficacy rates ranging from 20% to 80% depending on the study population.3 In addition, a substantial proportion of patients require repeat procedures. Pre-existing and/or progression of extra-pulmonary vein (PV) AF substrate likely plays a significant role in PVI non-responders and numerous substrate modification strategies have been investigated. However, given the large interindividual variability of electrical and anatomical AF substrate, achieving optimal patient outcomes requires individualised management. To this effect, the following key issues need to be addressed by clinicians and researchers: how to identify optimal candidates likely to benefit from AF ablation and which procedure endpoint(s) should be aimed at. This non-systematic review summarises the current literature regarding clinical practice, the most commonly proposed solutions and recent developments.
Optimal Candidate Selection
Clinical Stratification
AF Type
There is overwhelming evidence that the clinical AF phenotype is associated with post-ablation outcome, with progressively higher rates of arrhythmia recurrence for paroxysmal, persistent and long-standing persistent AF patients, respectively.3–5 Continuous duration of persistent AF and duration of AF history also correlate with post-ablation recurrence, and reports have suggested that ablation early in the course of the disease may prevent progression and even induce reverse atrial remodelling.6–9 As a result, substantial weight is generally accorded to AF type when evaluating the indication for AF ablation, as well as the ablation strategy itself. This is reflected in international recommendations.3–5
However, current definitions of AF types are derived from history-based arbitrary duration cut-offs rather than from prognostic studies and they lack objective validation with measured AF burden.10 Several large studies reported no association between AF type and recurrent AF after ablation when adjusting for other – likely stronger – predictors such as atrial fibrosis (see the imaging section). Therefore, while AF type is the most well established stratification tool for patient selection, it remains a relatively inaccurate predictor of ablation outcome as currently defined and other individual factors should also be considered.
AF Risk Factors
Numerous risk factors for arrhythmia recurrence after AF ablation have been reported and are summarised in Supplementary Material Table 1. There is considerable overlap between risk factors for recurrence after AF ablation and risk factors for new-onset AF and progression.11 This suggests that underlying disease progression is involved in AF recurrence after ablation. PV reconnection is traditionally considered to be a major cause of recurrence, but a recent meta-analysis found only a weak association, with 58% of AF-free patients also exhibiting PV reconnection.3,12 The importance of underlying disease progression is further supported by studies showing a decreased risk of post-ablation recurrence when treating modifiable AF risk factors such as obesity, hypertension or obstructive sleep apnoea.13
In contrast to this substantial literature, there are scarce data regarding clinical implementation of prognostic factors to guide patient selection for AF ablation. Likewise, few studies have examined hard outcomes following AF ablation. In this regard, it is crucial to consider the impact of sinus rhythm restoration on the course of the disease. For example, patients with progression risk factors may benefit from more aggressive management (with early ablation) to prevent disease progression, despite lower ablation success rates.14,15 Additionally, advances in ablation techniques and technology have led to fewer procedure-related complications, favourably shifting the benefit:harm ratio for increasingly broad populations.16,17
Based on current knowledge and ablation strategies, it may be reasonable to avoid ablation in patients estimated to have very low success probability based on substantial and unmodifiable risk factors. However, as discussed for heart failure (HF) in the next section, it will be crucial to determine if some of these patients may benefit from ablation despite lower success rates. Furthermore, observational data have shown risk factor management (RFM) to decrease the risk of post-ablation AF recurrence to rates comparable to low-risk patients.18 In the Aggressive Risk factor REduction STudy for Atrial Fibrillation and Implications for the Outcome of Ablation (ARREST-AF) observational cohort study,RFM including aggressive management of blood pressure, weight, lipids, glucose, sleep apnoea, smoking and alcohol consumption, was offered to AF patients undergoing CA.13 After an average 42-month follow-up, RFM was associated with a five-fold increase in arrhythmia-free survival compared with controls who underwent CA without structured RFM (87% versus 18%, respectively; p<0.001). While there are no prospective data investigating different ablation strategies in these populations, it should be noted that obstructive sleep apnoea has been associated with an increased prevalence of non-PV triggers.19
Heart Failure
The role of CA in the management of AF patients with HF is a complex and rapidly evolving field and a detailed discussion is beyond the scope of this review. Briefly, HF with preserved (HFpEF) or reduced ejection fraction (HFrEF) and AF frequently coexist and are mutual risk factors.20 HF has been reported as a predictor of arrhythmia recurrence after AF ablation.21 However, RCTs and subsequent meta-analyses have demonstrated the efficacy of AF ablation in patients with HFrEF, showing a nearly 50% reduction in mortality compared with medical treatment over follow-ups of up to 60 months.1,22 RCTs also demonstrated an improvement in left ventricular ejection fraction following CA compared with pharmacological treatment.1,22
Early intervention may bring additional benefit: in a meta-analysis of 26 studies including a total of 1,838 HFrEF patients with a mean 23-month follow-up, recurrence rates were lower when ablation was performed earlier after first diagnosis of AF or HF.15 Conclusive data are lacking regarding HFpEF, but observational data suggest similar efficacy.23 Upcoming RCTs such as the Randomized Ablation-based atrial Fibrillation rhythm control versus rate control Trial in patients with heart failure and high burden Atrial Fibrillation (RAFT-AF; NCT01420393) will likely shed additional light on the issue. It should be noted that the above study populations are likely to be biased towards low-risk HF patients. In this selected population, current international recommendations are to use similar indications for AF ablation as for patients without HF.3
Clinical Scores
Several scores based on clinical parameters have been designed and/or investigated to predict AF recurrence after ablation and are summarised in Supplementary Material Table 2. These multivariable scores remain modestly accurate, with area under the curve (AUC) values commonly in the 0.6–0.7 range, and have not been successfully implemented in prospective management strategies.
Imaging
Image-based predictors of post-ablation AF recurrence are summarised in Supplementary Material Table 3. While numerous parameters have been described, most fall into three conceptual categories: measures of left ventricular (LV) dysfunction (discussed above), measures of atrial structural remodelling, and measures of atrial mechanical remodelling.
Imaging Atrial Structural Remodelling
Atrial enlargement is a hallmark of atrial cardiomyopathy. A recent meta-analysis of observational studies confirmed that left atrial (LA) volume and LA volume index predicted the risk of AF recurrence after de novo CA regardless of the imaging modality (OR 1.032; 95% CI [1.012–1.052]; p=0.001).24 However, the effect was relatively small: the average difference between patients with and without recurrence was 0.8 ml for LA volume and 0.6 ml/m2 for LA volume index. Therefore, LA size alone does not appear to be a reliable prognostic indicator for clinical decision making.
Atrial tissue fibrosis is a prominent feature of atrial structural remodelling in AF patients and an established determinant of AF progression.25 The Salt Lake City group quantified LA fibrosis by delayed-enhancement MRI and demonstrated an independent association with arrhythmia recurrence following AF ablation.26-28 The authors proposed a staging system based on the extent of fibrosis with Utah stages I through IV corresponding to <10%, ≥10%–<20%, ≥20%–<30% and ≥30% of the LA wall volume, respectively. The cumulative incidence of recurrent arrhythmia at one year after ablation was 15%, 33%, 46% and 51% for stages I–IV, respectively.28 At five years,the cumulative incidence of recurrent arrhythmia was 53%, 66%, 72% and 87%, respectively.27 Interestingly, a secondary analysis from the efficacy of Delayed Enhancement-MRI-guided ablation vs. Conventional catheter Ablation of Atrial Fibrillation (DECAAF) cohort found that the extent of pre-ablation LA fibrosis not covered by ablation-induced scar at 3-month post-ablation MRI, or residual fibrosis, was strongly correlated with arrhythmia recurrence. This finding supports the notion that extra-PV AF substrate plays a significant role in AF recurrence after ablation.29 The on-going DECAAF II study will examine the efficacy of adjunctive fibrosis-guided ablation compared with PVI alone to prevent arrhythmia recurrence after AF ablation.
Imaging Atrial Mechanical Remodelling
The mechanical function of the LA consists of the reservoir, conduit and booster pump functions, the three of which are altered in AF and have been shown to predict AF recurrence after ablation.30–32 In a meta-analysis of eight prospective studies, LA strain by 2D speckle-tracking echocardiography (a measure of the booster pump function)predicted AF recurrence at an average 11.3 months post-ablation with 78% sensitivity, 75% specificity, mean AUC 0.798 (95% CI [0.70–0.94]).30 No study, however, has examined how these measures may be implemented in management strategies.
Circulating Biomarkers
A wide range of blood-based biomarkers have been associated with arrhythmia recurrence after AF ablation (Supplementary Material Table 4).33 These include markers of inflammation, myocardial injury, fibrosis and biomarkers associated with comorbidities. Similar to clinical risk factors, there is consistent overlap between markers associated with AF development/progression and markers associated with post-ablation recurrence. Selected biomarkers are discussed here based on novelty or their potential for clinical implementation.
Inflammation and Oxidative Stress
Various inflammatory biomarkers have been associated with AF;biomarkers associated with recurrence after ablation are summarised in Supplementary Material Table 4.34,35 It is likely that there are bidirectional causal relationships between AF and inflammation through local and systemic phenomena. Proposed mechanisms and mediators include endothelial injury, platelet activation, prothrombotic state, apoptosis, tissue injury, remodelling and fibrosis. How these markers may improve patient stratification and management remains to be determined prospectively.
Treatments targeting inflammation in conjunction with ablation have shown promising results. Deftereos et al.randomised 161 paroxysmal AF patients undergoing PVI to receive either a 3-month course of colchicine 0.5 mg twice daily or placebo.36 Colchicine was associated with a considerably lower risk of early AF recurrence at 3-month follow-up (OR 0.38; 95% CI [0.18–0.80]) and resulted in more pronounced reductions in C-reactive protein and interleukin-6 compared with placebo. In a second study with similar design,37 colchicine was associated with fewer AF recurrences compared with placebo after a 15-month median follow-up (31.1% versus 49.5%; OR 0.46; 95% CI [0.26–0.81]) and health-related quality of life improved accordingly.
Fibrosis and Extracellular Matrix Remodelling
Atrial fibrosis is a common endpoint of a variety of AF-promoting conditions, and AF itself induces remodelling and fibrosis.38 Fibrosis promotes AF through disruption of intermyocyte coupling, local conduction disturbances, arrhythmogenic fibroblast-myocyte interactions and heterogeneities in conduction properties and repolarisation.39 Circulating biomarkers of fibrosis associated with post-ablation AF recurrence are summarised in Supplementary Material Table 4.
Genetic Predictors of AF Recurrence
Genome-wide association studies and candidate gene studies have identified numerous polymorphisms associated with the risk of new-onset AF.40,41 A subset of these have been associated with AF recurrence after ablation, as well as with extra-PV triggers,pre-existent LA scars and LA diameter.42,43 In addition, genetic polymorphisms involved in inflammation,fibrosis and myocardial injury have been associated with post-ablation AF recurrence.44–46 While these data provide valuable insight into the underlying mechanisms, the modest predictive accuracy of genetic predictors has not translated into clinical implementation.
Electrocardiographic and Electrophysiological Parameters
Surface ECG and electrophysiology study parameters provide key information about the integrity and electrophysiological properties of the myocardium. Supplementary Material Table 5 lists examples of electrical predictors of AF recurrence after ablation.
Electrical Markers of Atrial Cardiomyopathy and Remodelling
Interatrial block (IAB), defined as a P wave ≥120 ms on any ECG lead, is a frequent condition generally resulting from impaired conduction within Bachmann’s region or adjacent atrial myocardium.47 IAB is associated with atrial fibrosis, LA enlargement and AF risk factors. In addition, IAB per se is a substrate for arrhythmia development and its association with supraventricular tachyarrhythmias is considered to be an arrhythmologic syndrome called Bayés syndrome.48 IAB is an established risk factor for AF recurrence after CA.48 In a recent meta-analysis of eight studies, the accuracy of IAB for the prediction of post-ablation AF recurrence (7–48 months of follow-up) was 72% pooled sensitivity, 58% pooled specificity, area under the summary ROC curve 0.66 (95% CI [0.62–0.70]).49
Low-voltage areas (LVAs) identified by electroanatomic mapping are a marker of fibrosis and correlate closely with late gadolinium enhancement at MRI.50,51 The extent of LVAs is an established predictor of recurrence after AF ablation.52,53 A causal relationship between LVAs and AF recurrence is supported by seemingly lower recurrence rates following PVI with adjunctive substrate modification targeting LVAs.54 However, the current literature is limited by the retrospective nature of most studies and the wide variability in ablation protocols; large RCTs are needed to determine the role of adjunctive LVA ablation in CA of paroxysmal and persistent AF.
Electrical Characteristics of AF
AF is characterised by disorganised atrial electrical activity with multiple simultaneously active wavefronts propagating with unstable, variable activation sequences. As a result, metrics used to characterise AF electrical activity have focused on quantifying the degree of organisation using measures of cycle length, amplitude, spectral organisation and entropy, several of which have been reported to predict arrhythmia recurrence after AF ablation (Supplementary Material Table 5). Regardless of the specific measure, greater AF disorganisation/complexity (for example short AF cycle length, high dominant frequency, low f wave amplitude, low spectral organisation, high entropy) is predictive of worse rhythm outcomes, likely because these parameters are related to a shorter atrial refractory period, more numerous simultaneous wavelets and atrial fibrosis.55 Moreover, AF seems to progressively organise within the 60–120 seconds preceding spontaneous termination, as shown by Alcaraz and Rieta’s sample entropy, a non-linear measure of signal regularity.56,57 Importantly, organisation parameters show a progressive decline in AF complexity in response to successful ablation compared with unsuccessful ablation, as shown with AF cycle length,dominant frequency (DF) and spectral organisation index.58–60 AF electrical complexity parameters show promise to refine patient selection and have been proposed to monitor the effect of ablation during stepwise procedures.59 The impact on long-term outcomes remains to be determined.
The extent to which AF complexity parameters could help identify ablation targets remains controversial. Atienza et al.performed biatrial DF mapping using 3D electroanatomic mapping in 50 consecutive patients (64% paroxysmal AF) and ablated sites of local maximum DF (DFmax), followed by PVI and post-ablation DF assessment.61 A significant reduction in DF in all chambers (left atrium, right atrium and coronary sinus [CS]) was associated with freedom from AF after an average 9-month follow-up, while patients with recurrent AF exhibited consistent DF reduction only in the LA. Successful ablation of all DFmax sites was a strong predictor of freedom from arrhythmia (78% versus 20%; p=0.001) compared to patients with residual untargeted DFmax sites. However, in a more recent multicentre RCT of 117 persistent AF patients, DF-based adjunctive substrate modification failed to improve outcomes compared to PVI alone.62 As a result of these conflicting data, international recommendations consider the strategy ‘of unknown usefulness’ (recommendation Class IIB).3
Functional Endpoints for AF Ablation
Non-inducibility
By analogy with other tachyarrhythmias in which persistent inducibility at procedure end is indicative of a poor arrhythmia prognosis, AF non-inducibility has been investigated as a tailored procedural endpoint for AF ablation. AF inducibility has been tested using two different modalities with different conceptual goals: rapid atrial pacing, or burst pacing, assesses sustainability, i.e. the capacity of the heart to maintain AF over time, thereby assessing the extent of AF-maintaining substrate, while isoproterenol infusion assesses the propensity of the heart to initiate AF, thereby identifying AF-triggering foci.
Non-inducibility by Atrial Burst Pacing
A normal heart should not sustain AF for more than a limited period of time – in the order of a few seconds for experimental models such as goats.63 In human studies, the majority of patients with healthy hearts did not sustain induced AF for ≥5 minutes,although the proportions varied with the induction protocol.64 Therefore, sustained induced AF is considered indicative of AF-maintaining substrate and, eventually, a marker of structural and electrophysiological remodelling. AF inducibility at procedure end (most commonly PVI alone) has been reported to predict AF recurrence in numerous studies.65,66 In addition, we recently demonstrated that a progression of AF inducibility from de novo AF ablation to repeat procedures was strongly associated with worse arrhythmia outcomes.67 Moreover, inducibility was shown to decline with additional ablation beyond PVI.58 As a result, inducibility has been used as a procedure endpoint in stepwise AF ablation protocols and recent evidence suggests that AF non-inducibility may be a better indicator of outcome than AF termination.58,68,69 Consistent with a relationship to AF substrate, AF inducibility has also been shown to be a strong predictor of new-onset AF in patients undergoing typical atrial flutter ablation.70 Finally, an ablation strategy aimed at achieving AF non-inducibility by electrogram-guided ablation lines has been found to improve arrhythmia outcomes in one RCT.71
However, the prognostic value of AF inducibility has not been reproduced consistently across studies.65,72 Moreover, the particular substrate modification strategy used to achieve AF non-inducibility is likely a critical determinant of outcome, yet none of the adjunctive ablation strategies investigated to date have an established benefit beyond PVI.3,73
The value of AF inducibility as a procedure endpoint is likely to be dependent on the induction protocol and on the cut-off duration used to define sustained AF; more data are needed to determine the limits of AF inducibility and sustainability in health and disease and how it relates to clinical outcomes after ablation.
Non-inducibility by Isoproterenol Infusion
High-dose isoproterenol infusion (typically up to 20–30 µg/min for ≥10 minutes) can be used to provoke non-PV triggers, allowing them to be located for ablation. While PV triggers are present in >90% of AF patients, including persistent and long-standing persistent AF,74 non-PV AF-triggering foci have also been reported in 11–32% of patients undergoing AF ablation.74–77 The most common locations include the superior vena cava, the CS, the ligament of Marshall, the posterior LA wall, the mitral valve annulus, the LA appendage and the crista terminalis/eustachian ridge.74–76,78 The presence of non-PV triggers at de novo AF ablation has been consistently reported as a predictor of AF recurrence.75,79–83 Crawford et al.reported AF inducibility by isoproterenol infusion to predict AF recurrence at 12 months with 33% sensitivity, 97% specificity and 83% accuracy.84 Recently, Hojo et al.assessed non-PV triggers in 216 patients (80% paroxysmal AF) who underwent de novo PVI followed by a second procedure at 6 months.85 The authors found a strong association between development of non-PV triggers and AF recurrence: 24.1% of patients with newly developed non-PV triggers had AF recurrence versus 7.4% of patients without newly developed non-PV triggers. Other observational studies have reported improved long-term outcomes after de novo AF ablation when non-PV triggers were identified by isoproterenol infusion and ablated.79 Of note, heterogeneous definitions of ‘significant’ non-PV triggers and ablation strategies have been used leading to heterogeneous results.86
Despite promising results, the lack of RCTs warrants caution. As a result, isoproterenol infusion and non-PV triggers ablation remains a Class IIB recommendation in the 2017 international expert consensus statement on catheter and surgical ablation of AF.3
Termination
The rationale for using termination as an endpoint of CA is to demonstrate efficient modification/elimination of the atrial substrate necessary to sustain AF. By analogy with other tachyarrhythmias, termination of a long-lasting arrhythmia during radiofrequency delivery can be attributed to the functional elimination of a critical driving mechanism. Of note, this endpoint has limited value for paroxysmal AF ablation since spontaneous termination may be fortuitous.
AF termination during stepwise ablation of persistent AF has been reported to predict AF recurrence in several studies.6,21,87–89 The mode of AF termination (directly to sinus rhythm versus via transformation into atrial tachycardia [AT]) was not predictive of recurrence in the majority of studies,but AF termination at index ablation seems associated with a greater proportion of recurrences in the form of AT relative to AF (with a lower total number of arrhythmia recurrences compared to patients without termination).65,87 This could be explained by differences in the lesion set between patients with termination and without termination, since studies investigating termination used stepwise ablation aimed at AF termination.
It should be noted, that other studies have shown conflicting results.65 The question was addressed in a substudy of the Substrate and Trigger Ablation for Reduction of Atrial Fibrillation II (STAR AF II) trial,a large RCT on persistent AF ablation primarily designed to compare the efficacy of PVI alone, PVI with electrogram-guided substrate modification (CFAE) and PVI with linear ablations.90,91 Acute AF termination was achieved in 5%, 40% and 17% of patients in each procedure group, respectively (p<0.001). There was no significant difference in 18-month AF-free survival between patients with and without termination (52.7% versus 42.4%; p=0.09), or between the three ablation strategies regardless of termination (59% of patients were AF-free at 18 months in the PVI group compared with 49% in the PVI + CFAE group and 46% in the PVI + linear ablation group; p=0.15). However, termination during the PVI step was predictive of AF-free survival (49.3% versus 35.7% in termination versus no termination, respectively; p=0.01). It is also noteworthy that AF termination may not indicate elimination of all AF drivers but only a subset which was active at the moment of ablation. Conversely, reverse atrial remodelling and/or trigger elimination may allow for more parsimonious ablation than the sometimes-extensive lesion set required to achieve acute termination. As suggested above, the role of termination per se has hardly been studied, given that the ablation strategies were guided by termination itself. In conclusion, AF termination seems to indicate a more favourable prognosis but alternatively may simply select a subgroup with a limited and ablation-sensitive set of driver mechanisms.
Summary
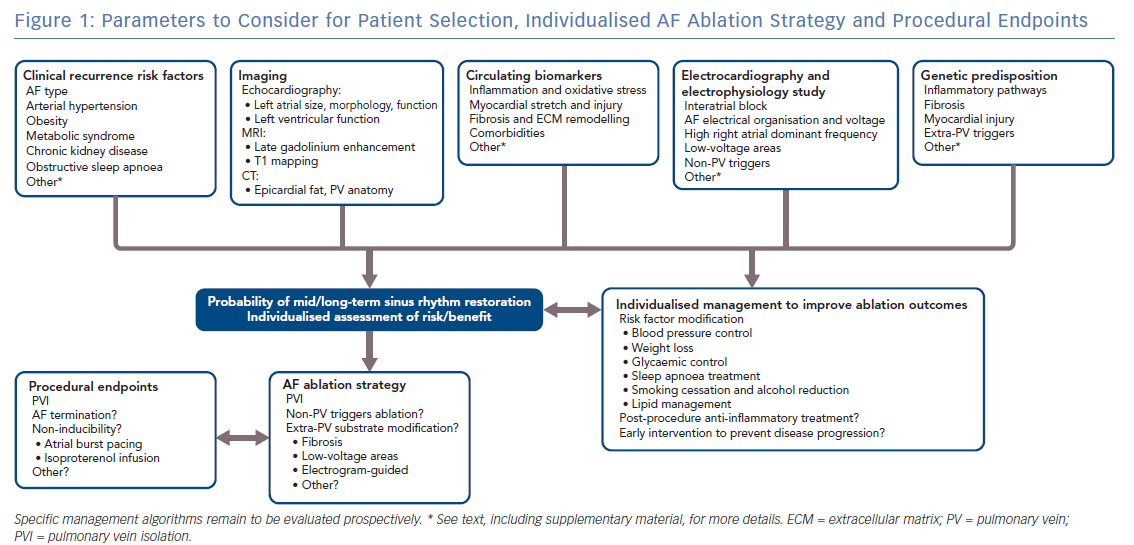
Figure 1 summarises the parameters to consider for patient selection, individualised AF ablation strategy and procedural endpoints.
Identified predictors of rhythm outcomes after AF ablation are significantly correlated with each other, probably because they measure the same underlying substrate. For example, while persistent AF, age and arterial hypertension are established indicators of poor prognosis, they were not significantly predictive of rhythm outcome after adjustment for fibrosis.92
A substantial limitation of the available evidence is its mostly observational nature. It should also be noted that some of the most frequently reported predictors of outcome also have a strong influence on the use (or lack thereof) of CA,which poses a substantial risk of bias in observational studies.93
Despite a considerable literature, no management algorithm for patient selection has arisen from these predictive tools to date. The predictive value of causal comorbidities is commonly limited by a lack of sensitivity, which is likely explained by the multifactorial nature of AF recurrence and progression.
Any discussion of the importance of patient selection and/or the choice of procedural endpoints necessarily has the greatest relevance in the context of a consistent ablation strategy. Obvious examples of this include the near uniform absence of a right atrial ablation strategy in most studies, and the lack of ablation within the CS in many studies. The lack of a consistent ablation lesion set with resulting demonstrable, reproducible and stable tissue alteration has also added a significant level of uncertainty to inferences derived from clinical studies. Nevertheless, slow but significant progress in achieving stable reproducible PV isolation and greater consistency in lesion creation has led to the acknowledgement of extra-PV mechanisms of AF sustenance and, in concert with lesion imaging technology such as MRI, may lead to objective standardisation of ablation lesion strategies and their resulting tissue effects.
The Supplementary Material for this article can be found here.
Clinical Perspective
- Clinical AF phenotype is an established stratification criterion to determine the indication to catheter ablation, but more individualised and objective selection is needed to improve outcomes.
- A number of predictors of AF ablation success based on clinical, electrophysiological, imaging and biological data may allow the identification of patients with very low probability of ablation success with current ablation strategies in these patients.
- In selected patients, such as those with HF, sinus rhythm restoration by early intervention may prevent disease progression and improve long-term outcomes.
- Individualised procedure endpoints, including AF termination and non-inducibility, have shown promise to achieve
improved arrhythmia outcomes via patient-specific lesion sets, but the lack of an established ablation strategy limits clinical inference. - Substrate-based ablation strategies based on delayed-enhancement MRI or selected electrophysiological surrogates are promising areas of progress.