X-rays used in interventional cardiology are proven (class I) carcinogens, and the electrophysiology community should make every effort to give “the right imaging exam, with the right dose, to the right patient”.1 This may be an effective strategy for the primary prevention of cancer for physicians, medical staff and patients (particularly children, young adults and women).2 The impact of X-rays on male and female reproductive health is well known.3 In men, radiation exposure could determine transient sperm count deterioration, resulting in long-lasting or permanent sterility, whereas in women, it can lead to alterations in the hypothalamic–pituitary axis function, affecting fertility and pregnancy outcomes.4 X-rays could lead to potential brain malignancies or long-lasting cognitive impairment, and microRNA dysregulation has been found to be related to certain forms of brain cancer and Alzheimer’s disease.5,6 Moreover, during interventional cardiology procedures, the left side, in particular, is usually exposed to higher radiation doses.7
Radiation exposure related to conventional radiofrequency catheter ablation carries small but non-negligible stochastic and deterministic effects on health.2 These effects are cumulative and potentially more harmful, and are worse in obese patients, who may need a far higher dose of radiation than people of normal weight, increasing their risk of cancer.2,8 Lead aprons can place considerable pressure on the spinal column, and wearing them for hours while standing has potentially detrimental consequences. The long-term use of lead aprons is known to result in orthopaedic disability, and as consequence, early retirement among physicians, technologists and nurses.9,10 Interventional cardiologists have reported neck and back pain, more time lost from work and a higher incidence of cervical disc herniations, as well as multiple-level disc disease.11 Therefore, several tools have recently been developed to facilitate arrhythmia mapping and ablation, including 3D electroanatomic mapping systems, magnetic navigation and intracardiac echocardiography, which significantly reduce the need for the fluoroscopic visualisation of catheters.12 Comparable long-term ablation outcomes, with clinical benefits for both patients and physicians, have been documented; the non-fluoroscopic approach is considered a feasible and safe alternative to fluoroscopy for arrhythmias ablation.12–15 The zero X-ray ablation approach is a milestone for cancer prevention in electrophysiological procedures.
The History of Zero X-ray Procedures
Transcatheter ablation has undergone impetuous advances in the past 25 years. Ablation mechanisms have been largely investigated, with electrophysiologists focusing on the link between anatomical aspects and electrophysiological properties.16–21 In the 2002 Pediatric Radiofrequency Ablation Registry, Kugler et al. compared ‘early’ and ‘recent’ eras, and found that the mean overall fluoroscopic time decreased by 21% in the paediatric population (from 50.9 ± 39.9 minutes in the early era to 40.1 ± 35.1 minutes in the recent era).22 However, X-ray doses were still high, and further improvements were necessary. In a study in the same year, Drago et al. used a 3D navigation system to eliminate fluoroscopic exposure to 21 paediatric patients.23 They demonstrated that ablation of right accessory pathways in children could be performed without fluoroscopy, using a single catheter with minimal amounts of radiofrequency applications, with a high success rate.23 There was a considerable reduction in the use of fluoroscopy after procedure 8, with a definitive and complete elimination of fluoroscopy from procedure 12 to procedure 21.23 Their study represented the start of the zero X-ray era.23 Two years later, in a study of 102 randomly selected patients referred for catheter ablation, Sporton et al. compared the routine use of electroanatomic imaging with that of a conventional fluoroscopically guided activation map, and documented a similar acute procedural success with both strategies.24
In 2005, the American College of Cardiology recommended that all catheterisation laboratories should adopt the ALARA (as low as reasonably achievable) principle for radiation doses, constituting a pivotal step towards minimising radiation use in invasive cardiology.25 In a multicentre randomised trial, Casella et al. compared a minimally fluoroscopic ablation with conventional fluoroscopic-guided ablation for supraventricular tachycardias in terms of ionising radiation exposure for 262 patients.26 They focused on the radiation exposure during electrophysiological procedures as non-negligible for both patients and medical staff, and found that a minimally fluoroscopic approach dramatically reduced the estimated risk of cancer incidence and mortality (96% reduction). They also found a reduction in estimated years of life lost and years of life affected, while retaining the safety and efficacy of procedures.26 Based on these findings, the electrophysiology community appealed to the industry to reduce costs and educate physicians to facilitate the implementation of this new electrophysiology for left atrial procedures.27 In their study, Raju et al. demonstrated that general anaesthesia, transoesophageal echocardiography and contact-force mapping catheters may all facilitate a minimised fluoroscopic approach among AF ablation patients.28 In this population, complete zero-fluoroscopy was possible in cases with patent foramen ovale (PFO), which was documented in 36% of patients.28
In 2016, we published a study of 442 consecutive patients who were referred for radiofrequency catheter ablation during a 5-year period (2009–2013).12 The patients were included in a retrospective observational study, where the first 145 patients (group 1) were treated only under fluoroscopic guidance; the other 297 patients (group 2) were treated with a non-conventional mapping system.12 The acute success rate did not differ between two groups, and there were no differences in either the procedure or complication rate. Moreover, fluoroscopic exposure in group 2 was significantly reduced compared with group 1 (14 ± 6 seconds versus 1,159 ± 833 seconds, p<.0001).12 We demonstrated how a near-zero radiation approach could lead to similar outcomes and significantly reduce or eliminate ionising radiation exposure. These reductions were achieved without altering the duration, or compromising the safety and effectiveness, of the procedure. In 2019, our group published the long-term outcomes of 266 patients who had undergone zero X-ray ablation, as no information was available on the long-term benefits.13 Patients were followed up for an average of 2.9 ±1.6 years, and a 100% rate of acute success was observed, with a complication rate of 0.8%; chronic success was achieved in 90.8% of cases, confirming that the complete elimination of fluoroscopy is advantageous and does not compromise results or patient safety.13
In a multicentre prospective study, the Zero Fluoro Study Group evaluated the determinants of zero-fluoroscopic ablation of 430 supraventricular tachycardias in 20 centres.15 The multivariable analysis identified the following predictors of zero-fluoroscopy: operator’s will, experience with >30 procedures, patient’s age and the type of arrhythmia (electrophysiological study and atrioventricular nodal re-entry tachycardia ablation having the highest probability of zero-fluoroscopy). The Zero Fluoro Study Group confirmed high safety and effectiveness profiles.15 In a recent study, Santoro et al. showed that catheter ablation can be performed without X-ray after an adequate learning curve.14 In 2011, they commenced an X-ray-minimisation programme using the CARTO System (Biosense Webster), with the intention to not aid X-ray unless strictly necessary. From 2011 to 2013, catheter ablations were performed without X-ray in 38.5% of cases, whereas from 2014 to 2017, there were no differences between the two groups in acute success, complications or duration for 525 procedures in 96.2% of cases (Table 1).14
Additional Advantages of Zero X-ray Ablation
In addition to the reduction in X-ray exposure, a non-conventional mapping system could offer several benefits. In the EnSite Precision cardiac mapping system (Abbott), catheter electrodes are detected and displayed based on the impedance measurements from three separate, orthogonal electrical fields, visualising any catheter within the system. The observable region, in particular, is wider, and these catheters can be visualised from the point of access (femoral vein or femoral artery) to the heart.29 The system’s drawbacks include a shift in geometry resulting from impedance changes, as lung volumes or total body fluid volumes change; shift could also occur due to patient perspiration, as well as changes in reference electrode contact.29 However, the benefits outweigh its limitations, such as the addition of magnetic capabilities of newer ablation and mapping catheters.30 The CARTO System functions by measuring magnetic fields, rather than electrical impedances, and is less prone to shift, but requires proprietary catheters (no catheter visualisation outside the magnetic field) and a longer time to draw a reasonable geometry.29 The Rhythmia mapping system (Boston Scientific) was specifically developed to support high-density/high-resolution mapping, and is a hybrid localisation system.31,32 In particular, magnetic tracking supports navigation-enabled catheters, providing maximum accuracy and efficiency (magnetic localisation ≤1 mm), whereas open architecture supports impedance-based tracking of non-navigation-enabled catheters for flexibility of choice (impedance localisation ≤2 mm).31,32 However, each of these systems functions in a unique manner, and due to rapid improvements, deficiencies are quickly disappearing.
Currently, mapping systems are able to locate the correct position of any pole at any time, and allow an accurate reconstruction of the geometry of both heart chambers and vessels, simplifying navigation and speeding up subsequent phases of the procedure.12 It is also helpful to understand the relationship between bipoles and cardiac anatomy, and between different structures and facilitating complex anatomy cases, continuously visualising two projections at the same time.33 Mapping systems allow visualisation of the catheters from the beginning to the end of the procedure.
In our laboratory, the first catheter inserted is a quadripolar/octopolar steerable catheter through the femoral vein, positioned in the coronary sinus (CS), whereas other diagnostic catheters are inserted and advanced using the previously reconstructed geometry as a guiding path. While moving the catheters, new anatomical points are typically collected in order to better define the boundaries of the areas of interest (both inferior and superior vena cava ostium, CS ostium, right atrial appendage, His bundle region). Integration with the continuous monitoring of intracavitary electrograms is useful during completely different ablation procedures.12 In cases of AP ablation, the location of the AP could be marked and still used to direct radiofrequency pulses in cases of ‘bump’, causing the AP to be no longer visible. In cases of atrioventricular nodal re-entrant tachycardia ablation, the procedure can also be simplified because of an optimal reconstruction of the Koch’s triangle, the anterior area of the CS and the proximity of the His bundle.12,13
In typical atrial flutter (AFL) cases, an activation map during CS stimulation may easily locate any gap along the isthmus ablation line.12,13 However, in atypical AFL, atrial tachycardia and ventricular tachycardia (VT) cases, mapping systems can be used to visualise arrhythmic circuits through activation maps, and to evaluate the electrical substrate through voltage maps.12,13 The high-density multi-electrode approach has significantly improved mapping of both complex atrial and VTs, with quicker and more accurate map creation.31,32 The additional advantages of a zero X-ray approach during pulmonary vein isolation should be considered. For example, it might be difficult to insert a standard spiral catheter in both lower pulmonary veins; in this case, it is possible to capture the spiral using a deflectable ablation catheter and to carry it in the lower vessels.
Our Daily Zero X-Ray Approach: Tips and Tricks
Ultrasound-guided Central Venous/Arterial Cannulation
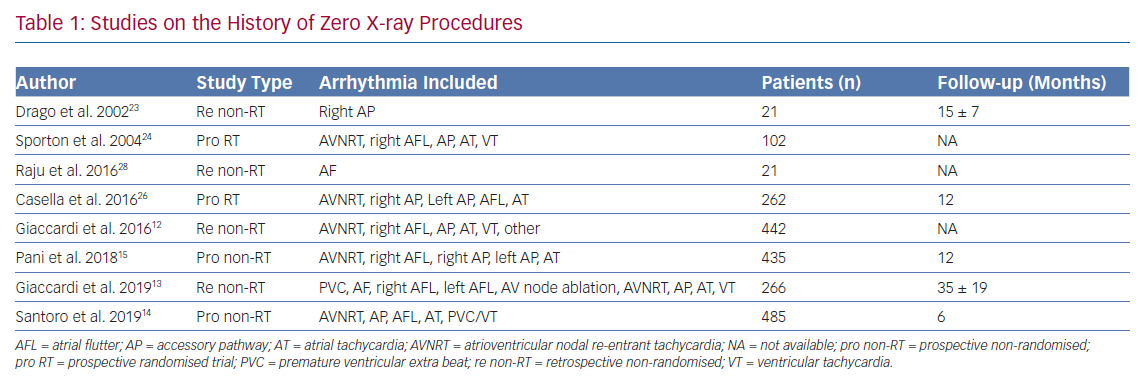
Ultrasound probes of 7–10 MHz are suitable for an ultrasound-guided central venous or arterial cannulation. Arteries are pulsatile and are identifiable, as they are difficult to compress. However, veins are non-pulsatile, are easily compressible and may distend when the patient performs Valsalva manoeuvre. A Doppler verification may also be used to confirm the anatomy. The superficial artery may overlie the femoral vein, and ultrasound imaging allows a differentiation of these structures, as well as an accurate puncture of the common femoral vein below the inguinal ligament during basic electrophysiology (Figure 1).
Zero X-ray Catheter Insertion: From the Groin Area to the Heart
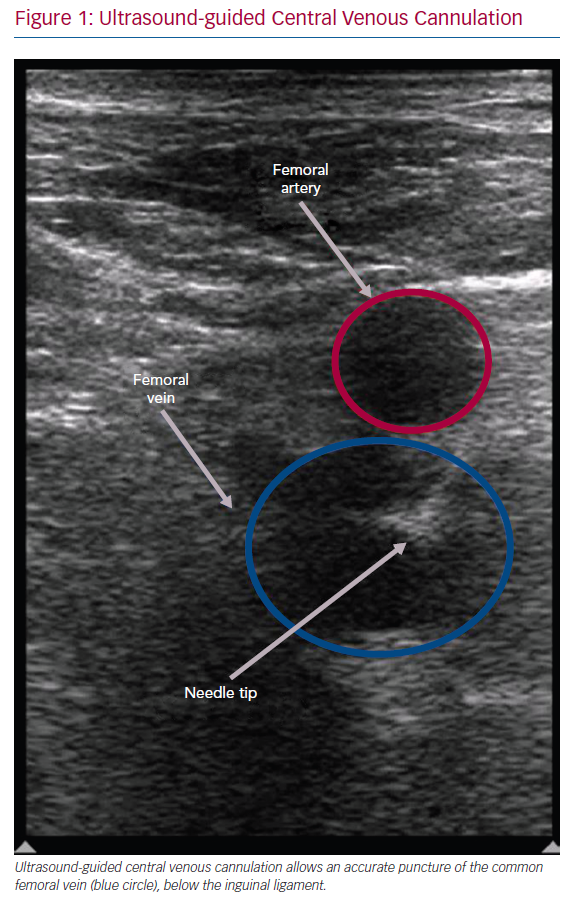
After obtaining femoral vein access with short sheaths, the catheter is threaded through the patient’s groin area to the heart. An anterior catheter direction is typically suggested to avoid collateral vessels. This direction may lead to the heart with no intermediate stops, as the anterior face of the inferior vena cava has no collateral vessels (Figure 2). The force that the catheter exerts on the blood vessel depends on the physician’s experience. Utilisation of ‘force-sensing’ ablation catheters may provide a real-time measure of the contact force between the catheter and the vessels, without the use of X-rays. Monitoring electrograms for an atrial signal will inform the operator when the catheter is in the right atrium.
Patent Foramen Ovale and Fossa Ovalis Mapping
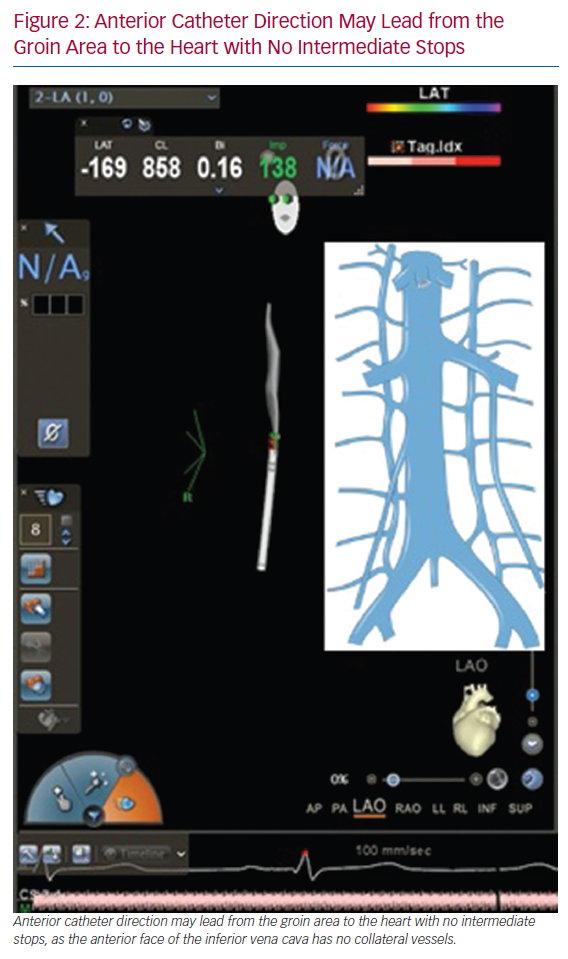
A PFO may be found after inserting the catheter into the right atrium and generating a 3D map with enough detail to identify both the septum and the fossa ovalis. Around 30% patients may have a PFO, allowing left-side procedures without the need for transseptal puncture (Figure 3).34–36
Transseptal Puncture: The Importance of an Adequate Learning Curve
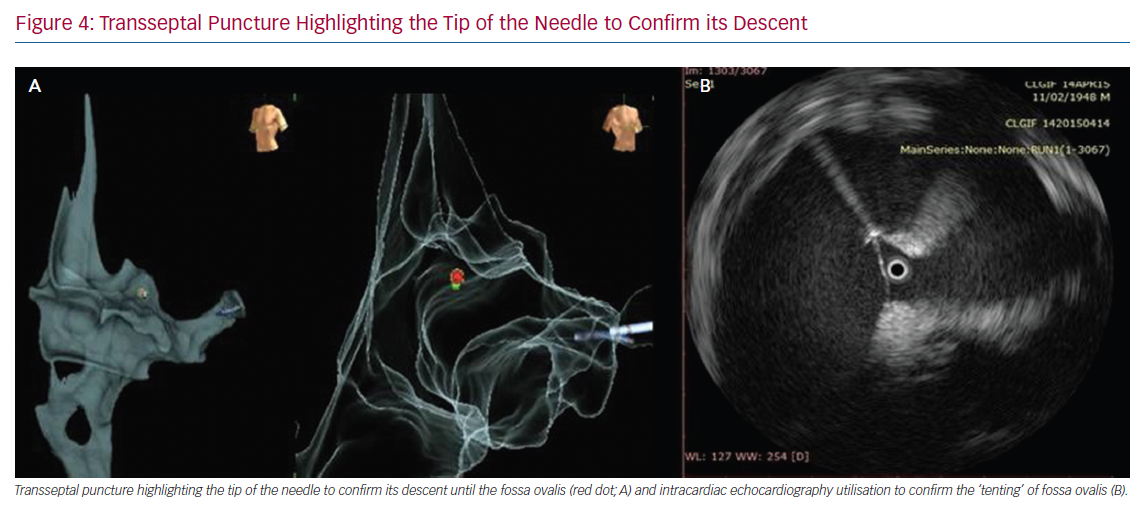
Transseptal puncture could cause potential issues when performing left-side zero X-ray procedures.37 In these cases, an in-depth understanding of cardiac anatomy, as well as high-level transoesophageal or intracardiac echocardiography (ICE) utilisation, is critical to reduce or minimise the use of X-rays. In the near-zero X-ray approach, the transseptal sheath may be visualised by inserting the ablation catheter via the long sheath and guiding it up to the superior vena cava (SVC) using the map as a guide. Once in the SVC, the sheath may be advanced slowly to cover the proximal ablation pole (which, for example, can be determined when the pole turns black on the CARTO System, or when a catheter deformation is documented on the Abbott system). The ablation catheter is then removed, and the physician may insert a dilator over a wire. The wire is then withdrawn and a transseptal needle is inserted 2 cm from the tip. At this point, the whole transseptal apparatus is withdrawn while looking at the transoesophageal or ICE images. The sheath could be observed to fall along the fossa ovalis, posteriorly directed towards the left-sided pulmonary veins. It is also possible to highlight the tip of the needle connecting the stylet to the system to confirm the descent of the needle tip until the fossa ovalis. Finally, when the needle reaches the fossa ovalis, it is possible to confirm its presence using transoesophageal or ICE images (Figure 4). Bidirectional guiding sheaths can be visualised on the mapping system and are important for eliminating sole dependence on fluoroscopy to determine their location.38
Contact-force Ablation Catheter Use During the Femoral Artery Approach
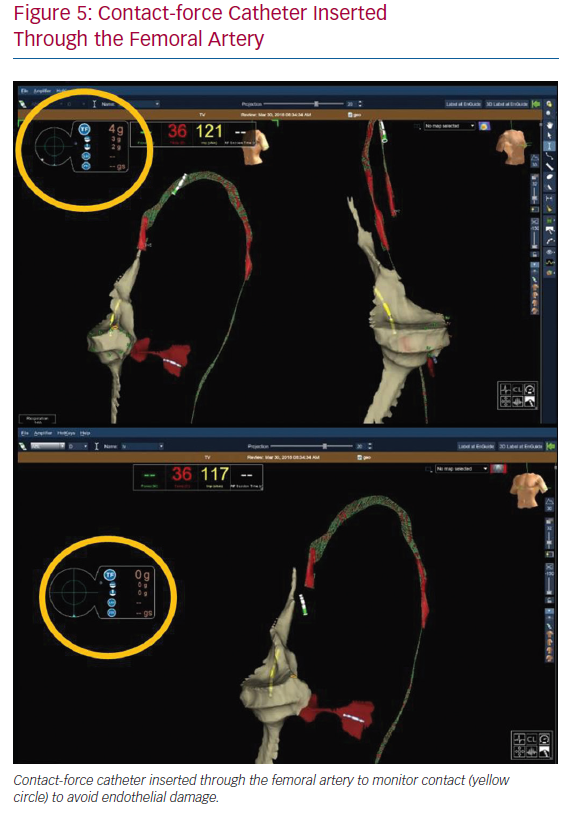
In the case of left chamber arrhythmic substrates, for which transseptal puncture is not required, the ablation catheter is inserted through the femoral artery from the start, as it is for the venous system. Modern ablation catheters enable monitoring of the contact force to avoid endothelial damage (Figure 5). Therefore, a contact-force catheter may not only be a therapeutic approach to arrhythmias but also a tool for achieving accurate characterisation of contact in the aortic vessel.
3D Imaging of the Oesophagus Before Pulmonary Vein Isolation Procedures
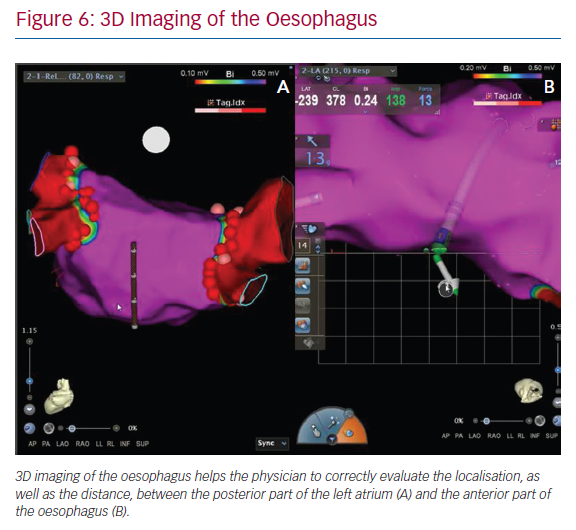
In our daily approach, the integration of an oesophageal tag into the electroanatomic left atrial map is usually performed during pulmonary vein isolations. The insertion of a quadripolar catheter into the oesophagus enables its 3D reconstruction and intraprocedural localisation. This approach can help physicians to correctly evaluate both the localisation of oesophagus and the distance between the posterior part of the left atrium and the anterior part of oesophagus (Figure 6). 3D imaging of the oesophagus may help to avoid an atrial-oesophageal fistula,39,40 which is a rare but lethal complication of AF ablation. While imaging modalities have improved, it is important for clinicians to maintain heightened awareness of this complication in post-ablation patients.
Our Perspectives
Innovative solutions have been found for reducing the dose per examination in all fields of medical imaging, including zero-fluoroscopy in electrophysiology, and the cancer- and non-cancer-related effects of medical radiation is currently the focus of the scientific community.2 However, awareness of risks remains the best protection against radiation exposure. More information about the harmful effects of ionising radiation is required in the form of antismoking, anti-alcohol and anti-obesity campaigns, as risk awareness may lead to a risk reduction. Zero X-ray ablation uses expensive technology and equipment. In 2013, Winkle et al. estimated a high cost per case for the use of magnetic navigation ablation.41
However, in the Near zerO fluoroscopic exPosure during catheter ablAtion of supRavenTricular arrhYthmias (NO-PARTY) multicentre randomised trial, Casella et al. found that a minimally fluoroscopic approach dramatically reduced the estimated risk of cancer incidence and mortality (96% reduction). They also found a reduction in estimated years of life lost and years of life affected, while retaining the safety and efficacy of procedures.26 Considering the impact of cancer on quality of life and the cost-effectiveness of this approach, as discussed in other studies, the intervention would be affordable at net cost between €1,151 and €1,918, which is the approximate cost of the mapping system.26,42
Therefore, in our daily practice, the physicians performing electrophysiological procedures should first ensure that exposure is as low as reasonably achievable without affecting quality of care. The zero X-ray approach is considered a reliable and safe alternative to fluoroscopy for tachyarrhythmia ablation.43 This method may yield potential clinical benefits in terms of reduction of ionising radiation exposure, as well as safe technical advantages. The benefits include no exposure of patients and staff to radiation, more precise definition or localisation of the mechanism of the arrhythmia, spatial display of catheters and arrhythmia activation, shorter procedure times (particularly in patients with complex arrhythmias) and easier access to ablation for certain populations (i.e. pregnant women and those undergoing radiation therapy). The long-term safety benefits of not using fluoroscopy have been documented, and the reduction in the use of X-rays has been achieved without compromising the duration, effectiveness and safety of the procedure.12–14 A planned approach may be necessary to define the optimal learning curve.14 The current role and next direction of cardiac magnetic resonance (CMR) in personalising arrhythmia management may be an important future point.44 CMR can determine precise and reproducible assessment of scar and ‘border zone’ volumes, as well as predict the location of re-entrant circuits within the scar to guide ablation.44 Detailed tissue characterisation may create personalised computer models to predict a patient’s risk of arrhythmia. Computational modelling provides a framework for the integration of experimental and clinical findings, and has emerged as essential mechanistic research of arrhythmias.45 Therefore, fluoroscopy may be used only in cases for troubleshooting, including transseptal puncture (considering the several tools we previously described to minimise the use of X-rays), potential peripheral vascular disease, previous lead implantation and epicardial ablation (especially for anterior/posterior pericardial access and for a potential coronarography prior to epicardial ablation). We have reached a new era in minimising X-ray radiation exposure, with new ideas and novel technologies still to be developed in the future.46,47
Conclusion
In ablation for arrhythmias, the zero X-ray approach is considered a feasible and safe alternative to fluoroscopy, which is only used in selected cases for troubleshooting. The non-fluoroscopic approach is a milestone for cancer prevention in ablation procedures. Awareness of radiation risk is a prerequisite to create a culture of respect for radiation hazard and a commitment to minimise exposure and to maximise protection.
Clinical Perspective
- The zero X-ray approach is a reliable and safe alternative to fluoroscopy for tachyarrhythmia ablation.
- The electrophysiologist should ensure that X-ray exposure is as low as reasonably achievable without sacrificing quality of care.
- A zero X-ray ablation may yield not only potential clinical benefits in terms of reduction of ionising radiation exposure, but also technical safe advantages.
- Fluoroscopy may be restricted to troubleshooting selected cases, since X-ray reductions are achieved without compromising the duration, effectiveness and safety of the procedure.
- The non-fluoroscopic approach represents a milestone for cancer prevention in ablation procedures.