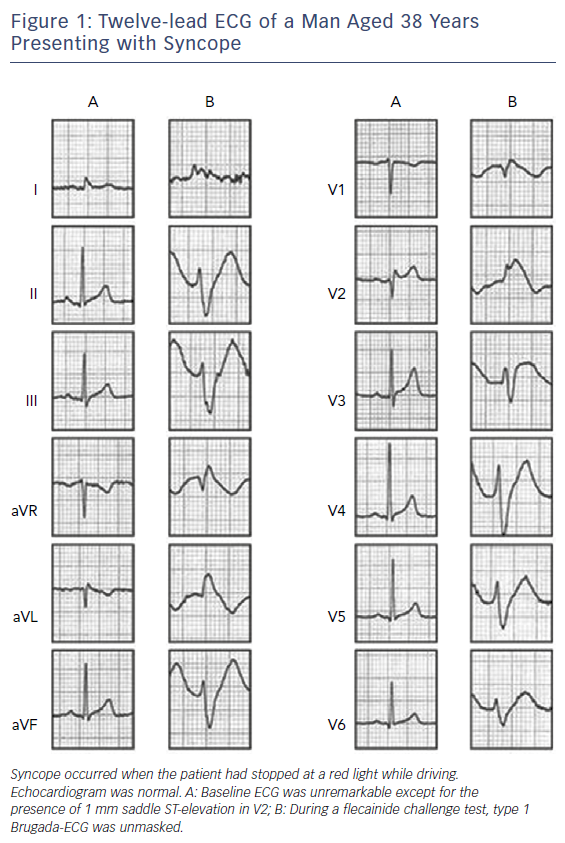
Brugada syndrome (BrS) is a cardiac disease caused by an inherited ion channelopathy. It was first described by the Brugada brothers in 19921 and is associated with a propensity to develop ventricular fibrillation (VF). Brugada syndrome is characterised by prominent J waves appearing as an ST segment elevation in the right precordial leads. In the latest guidelines, diagnosis of BrS constitutes ST elevation with type 1 morphology (coved) ≥2 mm in one or more leads among the right precordial leads V1 and V2, occurring either spontaneously or after intravenous administration of class I antiarrhythmic drugs.2–4 A recent expert consensus differs in the definition and recommends that when a type 1 ST segment elevation is unmasked using a sodium channel blocker, diagnosis of BrS should require that the patient also presents with one of the following: documented VF or polymorphic ventricular tachycardia (VT), syncope of probable arrhythmic cause, a family history of sudden cardiac death (SCD) at <45 years of age with negative autopsy, coved-type ECGs in family members, or nocturnal agonal respiration.5
Historically, BrS was considered to be inherited in an autosomal dominant inheritance with incomplete penetrance. There is growing evidence that presenting with BrS and the susceptibility to VF and SCD may not be due to a single mutation (classic Mendelian view) but rather to inheritance of multiple BrS susceptibility variants (oligogenic) acting in concert through one or more mechanistic pathways.5 Multiple mutations have been associated with the Brugada phenotype. These mutations cause either a decrease in the inward sodium or calcium current or an increase in the transient outward potassium channel current (Ito). Both result in an outward shift in the net transmembrane current active at the end of phase one of the right ventricular epicardial action potential (where Ito is most prominent).6–8
The guidelines recommend ICD implantation in patients with BrS who have survived a cardiac arrest, or have documented spontaneous sustained VT (class I).3,4 ICD implantation should also be considered in patients with BrS who have had a syncopal episode suspected to be caused by VT or VF (class IIa). In asymptomatic BrS individuals who have inducible sustained VF during programmed ventricular stimulation with two or three extrastimuli at two sites, ICD implantation is controversial and is a class IIb indication. ICDs are not recommended (class III) in reflex-mediated syncope or in asymptomatic patients.3,4 This recommendation is based on the low yearly event rate of 0.5 % found in asymptomatic Brugada patients,9,10 coupled with the high complication rate reported for ICDs in BrS.11–13 Aggregate rates of inappropriate shocks and lead failure have been reported to be as high as 37 and 29 %, respectively, at 10 years, including one death as a result of inappropriate ICD discharge resulting from lead failure.14,15 A near-fatal VF in BrS despite an implanted ICD has been reported, suggesting that lone therapy of ICD in cardiac arrest survivors with BrS is not without risks.16 The death of a BrS patient diagnosed after syncope (with an ajmaline provocation test), due to incessant VT and VF that developed during lead extraction procedure, has also been reported.17 In children and adolescents with BrS, high rates of inappropriate shocks and device-related complications were reported at 20 and 14 %, respectively.18 The option of subcutaneous ICD (S-ICD) for young patients with inherited arrhythmic syndromes who do not need pacing therapy is being increasingly used. A recent study found high rates of sensing screening failure in patients with BrS, due to high T wave voltages.19 Long-term clinical data are lacking at present on the utility of S-ICD in BrS.
A pharmacological therapy approach aimed at rebalancing the epicardial action potential in the right ventricle and normalising the action potential dome can prevent arrhythmogenesis in BrS, unlike a device therapy approach, which addresses only the symptoms of BrS without preventing the arrhythmias from occurring. Drug therapy in BrS has several utilities: first, in the acute management of arrhythmic storm; second, in prevention of arrhythmic events in patients with implanted ICD who require many shocks; and third, as an alternative to ICD implantation when the latter is contraindicated, not feasible (infants and young children), unaffordable, or refused by the patient. This review provides contemporary data gathered on all drugs effective in the therapy of BrS as well as ineffective or contraindicated antiarrhythmic drugs that should be avoided.
Class IA Antiarrhythmic Drugs
Quinidine
Quinidine’s beneficial effect in preventing arrhythmic events in BrS is mainly attributed to its significant Ito blocking property. It was shown that quinidine is effective in normalising the epicardial action potential dome and the ST segment and preventing phase-two re-entry and polymorphic VT in experimental models of BrS.6,20,21 The anticholinergic effect of quinidine may also contribute to its antiarrhythmic effect.8
Electrophysiologically Guided Quinidine Therapy
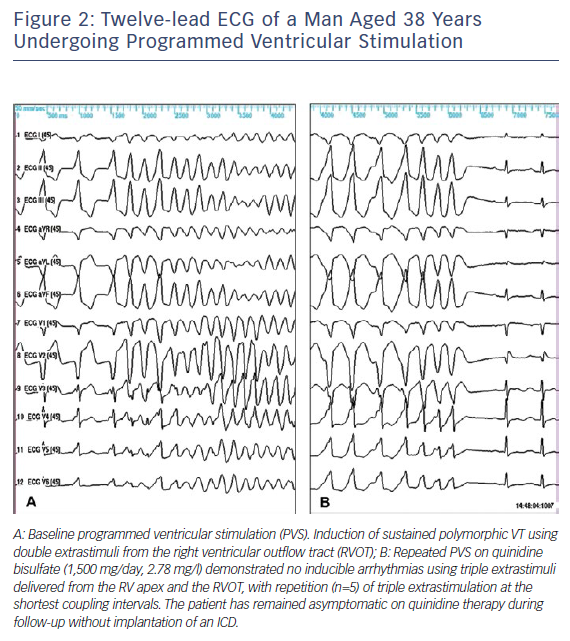
The first case showing the efficacy of oral quinidine in preventing inducible VF during electrophysiological study (EPS) and recurrent arrhythmias was reported in 1981 by Belhassen et al.22 in a young male with recurrent storms of idiopathic VF (IVF). This patient is still arrhythmia-free on quinidine treatment and with no implanted ICD after a follow-up of 39 years. Two of the five patients with IVF successfully treated with EP-guided class 1A antiarrhythmic drugs (AD) were later diagnosed as suffering from BrS.23 The first prospective series of 25 BrS patients treated with EP-guided therapy were also reported by Belhassen et al.24 Quinidine prevented VF inducibility in 22 (88 %) patients and no arrhythmic events were observed during follow-up in EP-quinidine responders treated by the medication. The latest study reporting the 33-year experience of the same group involved 96 patients with BrS (10 cardiac arrest survivors, 27 who presented with syncope and 59 who were asymptomatic). VF was induced in 66 (68.8 %) patients using an aggressive protocol of programmed ventricular stimulation (inducibility rates of 100 %, 74 % and 61 % in patients with cardiac arrest, syncope and no symptoms, respectively). All but six of the 66 patients with inducible VF underwent EPS on quinidine (n=54), disopyramide (n=2) or both (n=4). Two different formulations of quinidine were used during EP-guided therapy: quinidine bisulphate (QBS) (750–2000 mg daily) and hydroquinidine chlorhydrate (HQ) (600–900 mg daily). Fifty-four (90 %) patients were EP responders to more than one AD with similar efficacy rates (≈90 %) in all patient groups. After a mean follow-up of 113.3 ± 71.5 months, 92 patients were alive, whereas four had died from non-cardiac causes. No arrhythmic event occurred during class 1A AD therapy in any of the EP drug responders and in patients with no baseline inducible VF. Arrhythmic events occurred in only two cardiac arrest survivors treated with ICD alone but did not recur on quinidine. All cases of recurrent syncope (n=12) were attributed to a vasovagal (n=10) or nonarrhythmic mechanism (n=2). Class 1A AD resulted in a 38 % incidence of side-effects (mainly diarrhoea) that resolved after drug discontinuation. Sixty per cent of patients were compliant with the medication by the end of follow up.25 An illustrative example of the efficacy of quinidine in preventing VF induction is shown in Figures 1 and 2.
Based on these results, Belhassen et al.25 suggested that EP-guided therapy may be an excellent alternative to ICD therapy in selected patients who are committed to a life-long drug therapy and exhibit good tolerance to the medication.
Hermida et al.26 reported data of 31 asymptomatic BrS patients with inducible VF at baseline. They used HQ 600 mg daily in all their patients but two in whom they used 900 mg and found prevention of VT/VF inducibility in 76 % of their inducible patients. Syncope occurred in two of the 21 patients who received long-term (17 ± 13 months) HQ therapy. One syncope associated with QT interval prolongation and one unexplained syncope associated with probable noncompliance.26 Bouzeman et al.27 reported the results from two French centres, on 44 asymptomatic BrS patients. In 34 (77 %) of these patients, 600 mg HQ daily effectively prevented VF induction during a follow-up period of 3–6.2 years. VF occurred only in one patient in whom HQ was discontinued due to intolerance. Among the 10 other patients (22 %) who remained inducible and received ICD, none received appropriate therapy during a mean follow-up of 2–7.7 years. The overall annual rate of arrhythmic events was 1.04 %. One-third of patients experienced device-related complications.27 The higher rates of prevention of VF inducibility reported by Belhassen might be explained by the higher doses of HQ were used in their studies24,25,28 (900 mg daily versus 600 mg daily).26,27
It is noteworthy that in the current guidelines “quinidine should be considered (class IIa) in patients who qualify for an ICD but present a contraindication or refuse it, and in patients requiring treatment for supraventricular arrhythmias”.4
Empiric Quinidine Therapy for Arrhythmic Storm
Electric storms of VF represent the more malignant form of ventricular arrhythmias in patients with BrS, accounting for up to 10 % of the untreated patient population29 and up to 38 % of arrhythmic events in ICD patients.30 Isoproterenol infusion has been shown to be very effective in the acute management of arrhythmic storms in the setting of BrS.31 However, it can be administered only intravenously during a short period of time, and the arrhythmia frequently recurs upon drug discontinuation. Quinidine has been shown in multiple studies to successfully control arrhythmic storms as a single agent or in combination with isoproterenol.29,32–42 Anguera43 reported the effectiveness of quinidine in 29 BrS patients treated for secondary prevention due to arrhythmic storm (31 %) or frequent ICD shocks (69 %). Ten patients received QBS (mean dose 591 ± 239 mg/day), and 19 patients HQ (mean dose 697 ± 318 mg/day). After a mean period of 60 ± 41 months under quinidine treatment, 19 patients (66 %) remained free of appropriate ICD discharges. A significant reduction in total number of shocks and median number of shocks per patient was observed, from 203 to 41 shocks and from six shocks per patient IQR (4 to 9) to no shocks per patient IQR (0 to 2.5), p<0.0001, respectively. A total of 10 patients (34 %) experienced at least one recurrent shock (in four patients, shocks were related to a reduction in the dose of quinidine due to side-effects [n=2] or to temporary discontinuation of treatment by the patient [n=2]). During quinidine therapy, QT intervals corrected for heart rate increased by a mean of <10 % (413 ± 18 to 442 ± 35; p=0.001) without episodes of torsade de pointes. Side-effects appeared in five patients (17 %). The effectiveness of quinidine in controlling VF storm has also been described in children44,45 and pregnant women.46 The use of low-dose quinidine (<600 mg) in four patients with arrhythmic storms was reported by Márquez et al.47; quinidine prevented the recurrence of arrhythmic events in all patients.47
In the current guidelines, quinidine should be considered (class IIa indication) in BrS patients presenting with electrical storms and in patients implanted with an ICD who are experiencing repeated appropriate shocks.4
Empiric Quinidine Therapy for Asymptomatic Patients
The prophylactic use of empirical quinidine for asymptomatic patients with type 1 Brugada-ECG was first suggested by Viskin et al.48 Preliminary results in 19 patients who received empiric quinidine therapy were reported.49 No arrhythmic events occurred, and five patients (26 %) discontinued therapy due to side-effects. The administration of quinidine without further risk stratification to all asymptomatic patients with a type 1 Brugada-ECG is controversial. We routinely give quinidine to asymptomatic patients only in those who have inducible ventricular arrhythmias at EP testing.25 The recently published QUIDAM study: Hydroquinidine Therapy for the Management of Brugada Syndrome Patients at High Arrhythmic Risk50 is a prospective multicentre randomised (HQ versus placebo) double-blinded study with two 18-month crossover phases in high-risk patients with BrS and implanted with an ICD. Of the 50 patients enrolled, only 26 (52 %) fully completed both phases. Thirty-four (68 %) patients presented HQ-related side-effects, mainly gastrointestinal, which led to discontinuation of the therapy in 13 (26 %). During the 36-month follow-up period, two arrhythmic events occurred under placebo therapy (one appropriate ICD shock [0.97 % event per year] and one self-terminating VF). In addition, one inappropriate ICD shock occurred under placebo therapy. No arrhythmic events were reported under HQ therapy. The authors concluded that although HQ seems to be effective in preventing life-threatening arrhythmic events, it could not be an alternative for ICD implantation due to its frequent side-effects limiting compliance to the drug. This study, although well structured, was underpowered to prove the efficacy of HQ due to the small number of patients enrolled and even smaller number of patients who completed both phases. In addition, the very high incidence of side-effects reported was inconsistent with previous reports.25,27 In his editorial comment on the paper by Andorin et al., Belhassen51 pointed to the study’s patient population being mainly at intermediate and low arrhythmic risk, which explains the difficulty of assessing the efficacy of quinidine therapy, even more so with a small cohort population. In addition, he postulated that the high rate of side effects (mainly diarrhoea) requiring drug discontinuation could have been related to patients’ reluctance to continue the trial while they felt fully protected by the implanted ICDs.
Due to the low incidence of arrhythmic events in asymptomatic BrS patients, a large-scale double-blinded trial is needed for further validation of the efficacy of HQ in asymptomatic BrS patients. A prospective registry of empiric quinidine for asymptomatic Brugada syndrome has been established.48
Low-dose Quinidine
To decrease the frequency of side-effects associated with quinidine, administration of lower doses of the drug have been attempted. Márquez et al.47 reported a small series of six BrS patients, in whom doses of quinidine or hydroquinidine (<600 mg) prevented the recurrence of arrhythmic events in all patients without side-effects during a median follow-up of four years. In the literature review, 14 additional patients treated with <600 mg of quinidine were found. Quinidine was well tolerated and associated with acute and long-term arrhythmia control in 85 % of cases. In four patients who stopped taking the medication, recurrent arrhythmias occurred, which were successfully controlled after treatment was reinitiated.47 Hasegawa reported the normalisation of J waves and coved-type ST-segment elevation with VF suppression in a patient who presented with a VF storm after daily administration of 300 mg quinidine and a follow-up of 20 months.52 These data suggest that low doses of quinidine, which are generally well tolerated, may be useful in controlling arrhythmias. Further studies of larger scale are needed to validate the efficacy and safety of low-dose HQ.
Limited Worldwide Commercial Availability of the Drug
A major limitation for the wide use of quinidine in the world is its lack of accessibility.53,54 Viskin et al.54 asked a total of 273 physicians from 131 countries regarding the availability of quinidine in their countries. Quinidine was readily available in 19 countries (14 %), not accessible in 99 countries (76 %), and available only through specific regulatory processes that require 4 to 90 days for completion in 13 countries (10 %). Viskin et al.54 were able to gather information concerning 22 patients who had serious arrhythmias probably related (10 cases) or possibly related (12 cases) to the absence of quinidine, including two fatalities possibly attributable to the unavailability of quinidine.
Disopyramide
Disopyramide, a class IA antiarrhythmic drug, exhibits a moderate use-dependent block of INa and moderate block of Ito. In addition, disopyramide has been reported to decrease the inhomogeneity between infarcted area and normal myocardium refractory period, while lengthening the ventricular refractory period, hence decreasing the chance for phase two re-entry.55 These effects can explain its efficacy in BrS. Even though the effect of disopyramide in slightly augmenting ST elevation in BrS has been described in small studies, it has not been associated with development of premature ventricular complexes or VT/VF.56,57
Miyazaki et al.57 reported the use of disopyramide in three BrS patients, and found that 50 mg of IV disopyramide resulted in augmentation of ST elevation. However, in one patient, the combined use of intravenous and oral disopyramide resulted in VF non-inducibility.
Belhassen et al.25 reported that the use of disopyramide at a mean dose of 500 ± 71 mg (300–600) prevented VF induction during EPS in three (50 %) of the six patients tested; two of the three disopyramide non-responders responded to BSQ. The efficacy of disopyramide in VF suppression was described in case reports of BrS patients with VF storm.58,59 VF suppression occurred despite exacerbation of ST segment elevation, suggesting the efficacy of disopyramide in suppressing VF might not correlate with ECG normalisation.59
Current data suggests that disopyramide may be useful in controlling ventricular arrhythmias in BrS; however, larger studies are needed to fully characterise the effect of oral and intravenous disopyramide in BrS.
Quinine Sulphate
Quinine is the diastereomer of quinidine that has been used to treat malaria. In dogs, it has similar effects to quinidine on conduction time but does not prolong epicardial repolarisation time or ventricular refractoriness. In experimental models, studies have demonstrated the antiarrhythmic effect of quinine in suppressing VF thresholds.60 In humans, quinine is effective in suppressing both spontaneous and inducible ventricular arrhythmias without the proarrhythmic potential of QT prolongation, torsade de pointes, or heart block.61 In one case report,32 monotherapy with quinine sulphate was effective in preventing electrical storm and recurrent ICD shocks. This medication also resulted in normalisation of ST-segment elevation.32 The use of intravenous quinine was reported for the acute management of an arrhythmic storm in a 10-year-old child.62
Beta Adrenergic Agonists
A diminished inward current of Ica combined with the prominent Ito current in the epicardium results in an intensified repolarisation of the RV subepicardium, where Ito activity is most pronounced. When phase one is repolarised beyond the voltage range at which L-type Ca2+ channels activate, the Ca2+ channels fail to activate, resulting in loss of the action potential dome.7 Beta adrenergic agents, such as isoproterenol, denopamine or orciprenaline, augment L-type calcium channels, and this is the basis for their usefulness in controlling VF storms in BrS.29,33,63 Isoproterenol has been shown to be effective in controlling VF storm either as a lone agent or in combination with quinidine.29,37,38,40,46,57,64–70 The effectiveness of isoproterenol in controlling VF storm was also described in children45,62,71 and pregnant women.46,66 Current guidelines recommend the administration of isoproterenol for BrS patients in VF storm (class IIA).3–5
Phosphodiesterase Inhibitors
Cilostazol is a phosphodiesterase III inhibitor with therapeutic focus on cyclic adenosine monophosphate (cAMP). It inhibits platelet aggregation and is a direct arterial vasodilator. It increases cellular cAMP levels and L-type calcium currents, and, like isoproterenol, counteracts Ito, resulting in attenuation or abolishment of the electrical inhomogeneity of action potentials. The reduced electrical inhomogeneity of action potentials would prevent phase two re-entry and subsequent VF, thereby leading to the diminution or disappearance of coved-type ST-segment elevation or J waves. The successful use of cilostazol 200 mg daily in preventing VF storm in BrS, resulting in abolition of J waves and transformation of coved-type ST-segment elevation to saddleback-type has been reported in several cases.52,72–77 However, the failure of cilostazol 200 mg daily in preventing a VF storm has also been reported.32, 80 Worthy of mention, cilostazol can cause symptomatic palpitations,77 and its long-term effects have not been reported. The combined use of cilostazol and bepridil has been reported to attenuate cilostazol induced palpitations (see below).77
Milrinone is another phosphodiesterase III inhibitor recently identified as a more potent alternative to cilostazol in suppressing ST elevation and arrhythmogenesis in an experimental model of BrS.76,79 So far, no clinical data have been reported in humans.
Bepridil
Bepridil is a long-acting, non-selective, amine calcium channel blocker previously used for its significant anti-anginal activity. Its antiarrhythmic effects have not been fully characterised. Bepridil has demonstrated multiple effects on cardiac ion channel currents. Effects that appear to be relevant are block of Ito, augmentation of INa via up-regulation of the channels80 and prolongation of QT at slow rates, thus increasing the slope of QT-RR.81,82
The effectiveness of bepridil in preventing VF, usually in combination with other drugs, has been described in several small studies and case reports.29,70,77,81–83 In a study of seven patients with repetitive VF episodes, Murakami et al.84 reported that the use of bepridil 100–200 mg daily prevented recurrence of VF along with improvement of ST elevation and of low-amplitude signals in four patients with BrS with the SCN5A mutation but not in those without this mutation. Bepridil was effective in the long-term prevention of VF in the highest-risk patients with electrical storms who demonstrated early repolarisation in addition to BrS.85 Addition of bepridil attenuated cilostazol-induced palpitations by eliminating sinus tachycardia, and maintained the suppressive effect of cilostazol against VF in a study of seven patients with J wave-syndrome-associated recurrent ICD shocks (five BrS patients).77 This effect of bepridil may be due to its effect to block If86 as well as its Ica-blocking effect.7 Currently, bepridil is available only in Japan.
Traditional Chinese Medicine
Wenxin Keli
Wenxin Keli (WK) is a Chinese herb extract, reported to be effective in the treatment of atrial and ventricular cardiac arrhythmias.87–93 WK has been reported to block Ito, sodium current (INa) and L-type calcium current (Ica) in rat and rabbit ventricular cardiomyocytes.94 A recent study found that WK, particularly in combination with low-dose quinidine (5 μM), effectively suppresses arrhythmogenesis in an experimental model of BrS via inhibition of Ito and indirect adrenergic sympathomimetic effects.95
Dimethyl Lithospermate B
Dimethyl lithospermate B, an extract of danshen, a traditional Chinese herbal remedy, has been reported to slow inactivation of INa, thus increasing INa during the early phases of the action potential and suppressing arrhythmogenesis in experimental models of BrS.96 No clinical data are available yet.
Ineffective Drugs
Amiodarone
Amiodarone has not been shown to be effective in controlling arrhythmias in BrS.1,97,98 Moreover, there are a few case reports in which acute amiodarone infusion unmasked a Brugada phenotype ECG pattern and aggravated a VF storm.99–103 Amiodarone is predominantly a potassium ion channel–blocking agent, but has been shown in vitro to have sodium ion channel-blocking properties,104,105 especially in the acute phase of its administration. This effect provides a plausible scientific basis for unmasking BrS ECG pattern.
Beta Blockers and Calcium Channel Blockers
In the Defibrillator Versus beta-Blockers for Unexplained Death in Thailand (DEBUT) trial,106 Nademanee et al. compared the use of defibrillators versus beta-blockers in sudden unexplained death syndrome survivors. Approximately 55–60 % of patients had ECG features of BrS. Eighty-six patients were randomised (in two study phases) to receive an ICD or propranolol. During the 3-year follow-up period of the main trial, there were four deaths; all occurred in the beta-blocker group (p=0.02). Seven subjects in the ICD arm had recurrent VF, and all were effectively treated by the ICD. In total (both from the pilot study and the main trial), there were seven deaths (18 %) in the beta-blocker group and no death in the ICD group.106 Beta-blockers and calcium channel blockers are known to increase ST-segment elevation57,107 and to cause initiation of VF.108,109 Experimental data suggest that beta blockers and calcium channel blockers decrease inward calcium current and cause an outward shift in current at the end of phase one of the action potential. This creates a transmural voltage gradient, leading to ST-segment elevation and ventricular arrhythmias.109 Some clinical studies supporting these experimental data have been reported with small samples. In two case reports, severe propranolol toxicity was reported to result in the Brugada ECG pattern in an otherwise healthy individual or to unmask BrS.109,110 This can be explained by the fact that at high doses, propranolol binds to the cardiac sodium channels and inhibits sodium uptake. Kasanuki et al.112 reported that VF induction was exacerbated by intravenous injection of propranolol in BrS patients. However, in a recent study of 29 patients receiving a beta-blocker (22 patients) or calcium channel blocker (eight patients) for more than one year for the treatment of comorbidities, Kamakura et al.113 found that the long-term oral intake of these medications at normal dosage range was not associated with the aggravation of ECG parameters and clinical outcome in patients with BrS; thus they concluded that the use of these medications is acceptable under careful observation.113
Contraindicated Drugs
Class IA (ajmaline, procainamide) and class IC (flecainide, propafenone and pilsicainide) sodium channel blockers drugs are known to unmask type I ST-segment elevation in the ECG and induce cardiac arrhythmias in BrS.56,85,97,109,114–123 The occurrence of cardiac arrhythmias during sodium channel blockers challenge ranges from 0–17.8 %.121 Hence, these drugs are contraindicated in the therapy of BrS.
Conclusion
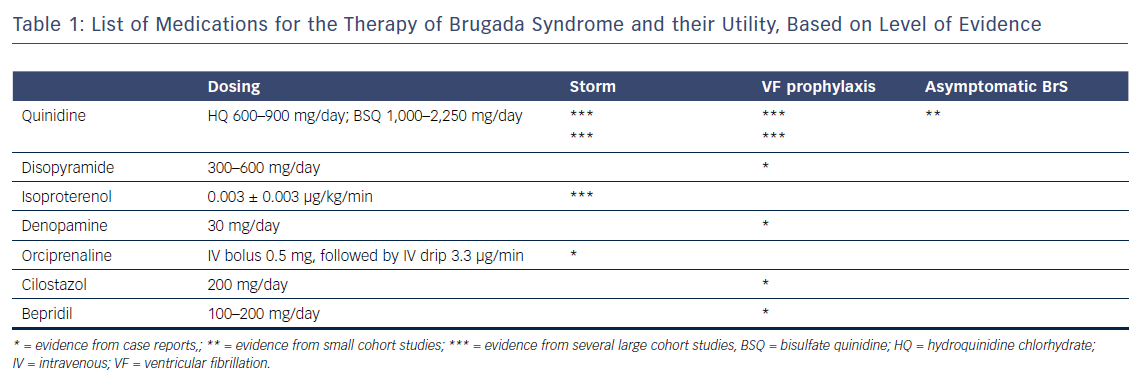
This review provides contemporary data on each of the drugs effective in the therapy of BrS. A pharmacological approach to therapy is aimed at rebalancing the epicardial action potential in the right ventricle, normalising the ECG abnormalities and preventing cardiac arrhythmias. Regardless of whether or not an ICD is implanted, prevention of recurrent arrhythmic events, especially in the high-risk population of cardiac arrest survivors, should be considered. Quinidine is the most extensively studied medication with proven efficacy in successfully controlling and preventing arrhythmic events in BrS. It is the authors’ opinion that this medication is an alternative to ICD therapy in all types of BrS patients who have fulfilled the strict conditions detailed elsewhere.124 Table 1 provides a summary of the drugs effective in BrS, their recommended dosing and their utility.