Ventricular arrhythmias remain a major contributor to cardiac morbidity and mortality worldwide, despite ongoing research and implementation of novel therapeutic interventions. Modern management of patients with ventricular arrhythmias requires a multidisciplinary team approach, especially in complex presentations with a background of multiple medical comorbidities.1,2 Such teams may include cardiac electrophysiologists (EP), heart failure specialists, general cardiologists and cardiac surgeons, as well as nurses, psychologists and primary care physicians. In emergency presentations with sustained or recurrent ventricular tachycardia (VT) or multiple ICD shocks (‘VT storm’), additional involvement of emergency physicians, intensivists, cardiac anaesthetists and coronary care unit (CCU) staff may be required.
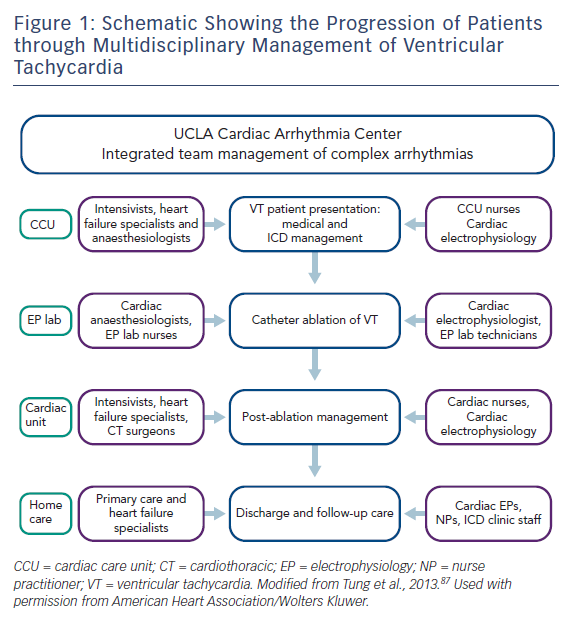
Antiarrhythmic medications, ICD implantation and catheter ablation are the cornerstones of current VT management. Recently, catheter ablation has gained a prominent and earlier role in the management of patients with VT. Caring for patients undergoing catheter ablation of VT in dedicated units with integrated multidisciplinary care has been shown to lead to improved outcomes (Figure 1).3
In this article, we review the team approach and process of managing VT patients. The patient’s journey often begins in the clinic or emergency room, proceeding to some or all of the following areas: coronary care unit, cardiac catheterisation and EP labs, cardiac operating room, recovery unit, rehab unit to discharge home.
At each step, multiple teams need to be involved and coordinated to optimise the patient’s care. In our centre, the primary cardiologist coordinates the patient’s care with close involvement of the cardiac EP team throughout the hospital stay. We will first consider the patient presenting with sustained VT, then reviewing the special situation of VT storm or incessant VT.
Sustained Ventricular Tachycardia
Patients with VT may present with palpitations, syncope or ICD shocks where a device is present; in addition, their clinical status and haemodynamic stability will vary. Patients may present to primary or emergency care, or in general cardiology outpatient clinics. Therefore, the recognition of VT through the presence of a wide complex tachycardia on ECG is important for frontline caregivers. Wide complex tachycardias are most often VT and should be treated as such unless proven otherwise. The differential includes supraventricular tachycardia (SVT) with aberrancy, with abnormal baseline QRS, drug effects or electrolyte imbalances, and ventricular pacing. Multiple algorithms for ECG diagnosis of VT have been proposed, which have been well described elsewhere.4 VT algorithms are complex, leading to difficulties in application. With this in mind, simplified algorithms requiring only single-lead measurements such as the Vereckei criteria5 and Pava criteria6 have been developed, although their overall accuracy may be reduced.
Initial Management and Antiarrhythmic Therapy
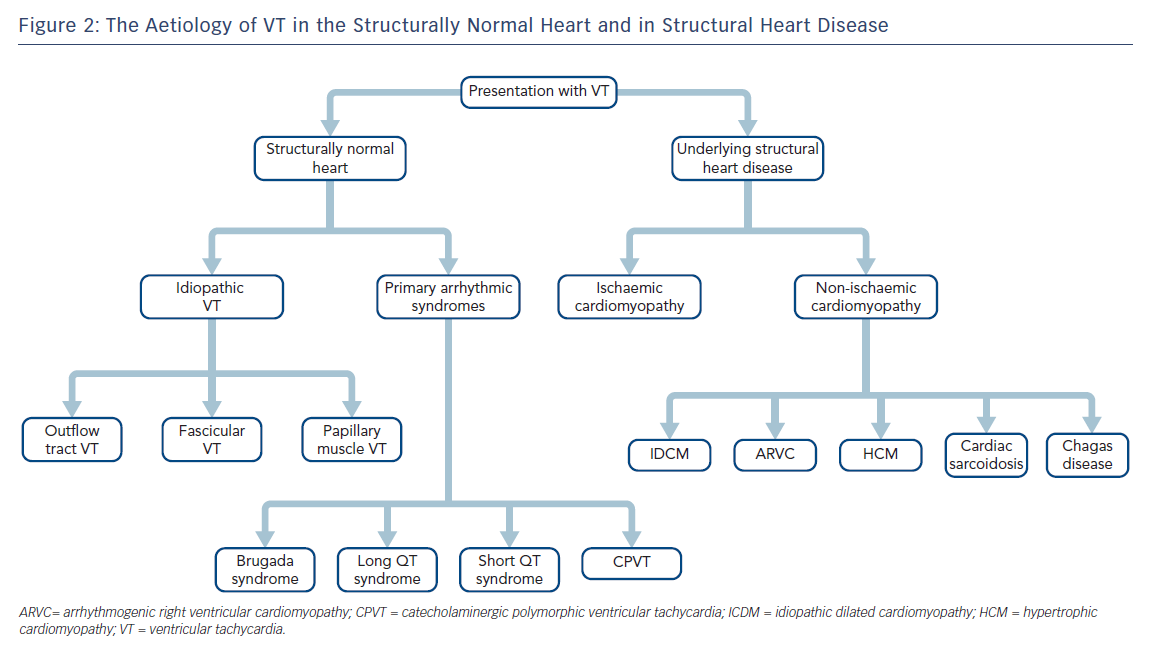
It is most useful to approach the initial investigation and management of VT by its causes, broadly divided into those occurring in structurally normal hearts and those in the context of structural heart disease (SHD; Figure 2).
In haemodynamically unstable sustained VT, the priority is stabilisation and electrical cardioversion. In haemodynamically stable VT, a history, an examination and a 12-lead ECG should be obtained, and treatment with antiarrhythmic medications initiated. If the VT morphology suggests idiopathic outflow tract VT (left bundle pattern with inferior axis), IV beta-blockers may terminate the arrhythmia. Otherwise, IV amiodarone is considered the most effective first choice for pharmacological management.1,7 IV procainamide can be safely administered at a slow infusion rate, with efficacy for VT termination of 60–80 %, superior to amiodarone.8 Lidocaine has a lower efficacy of around 15–30 %, but is commonly used.7,9 It is important to closely monitor patients while administering antiarrhythmics for haemodynamically stable VT, as hypotension is a side-effect of both amiodarone and procainamide, which can lead to worsening of symptoms and haemodynamically unstable VT: in these cases, prompt sedation and cardioversion is required.
Investigations and Imaging
After acute termination, further investigation into the underlying cause is necessary. A thorough history and examination will help identify any risk factors and potential causes for SHD or primary arrhythmic syndromes. Review of the baseline 12-lead ECG may provide evidence of underlying myocardial disease or arrhythmia syndromes (Wolff-Parkinson-White, long QT, Brugada). A 12-lead ECG of the clinical VT is of great value, as this can indicate aetiology and exit site of VT.10 In rare cases, invasive electrophysiological study may be required to confirm diagnosis.1 Coronary angiography should be undertaken in all patients with recurrent VT to define the coronary anatomy.
In patients with ICDs, device interrogation should be undertaken as soon as the patient is stabilised. The device log can provide a full history of the number of VT episodes, therapies delivered and whether shocks are appropriate, inappropriate or even phantom in nature. Additional ECG investigations, such as signal-averaged ECG, can be useful in detection of late potentials and diagnosis of specific cardiomyopathies, such as arrhythmogenic right ventricular cardiomyopathy (ARVC).
Imaging is also indicated to detect the presence of SHD. In patients with known SHD, it may be useful to perform repeat imaging to assess current function and progression of disease. Echocardiography is the first-line investigation and can also provide an acute estimate of the left ventricular ejection fraction (LVEF). Where there is diagnostic uncertainty, contrast-enhanced cardiac MRI (CMR) can give further clarity; in addition, a significant proportion of apparently normal hearts at echocardiography may subsequently display structural disease on CMR imaging.11 Detection of late gadolinium enhancement (LGE) has been shown to correlate with the risk of arrhythmia and sudden cardiac death, and areas of scarring identified on CMR imaging have been demonstrated to correlate with areas of scarring on electroanatomic mapping and histopathology.12,13 CMR imaging aids diagnosis, risk stratification and planning for ablation. It can reveal significant details of complex scars, which can help focus mapping and ablation efforts.14
However, CMR imaging has been an investigation that has previously been contraindicated in many patients with ventricular arrhythmias, SHD and heart failure, where the prevalence of implanted cardiac devices is high. Specific concerns have included device lead heating causing thermal myocardial injury, arrhythmias, lead failure, and device failure or malfunction.15 To overcome this, MRI-conditional devices and imaging protocols for extra-thoracic studies with non-conditional devices have been developed over recent years with demonstration of safety.16 This involves assessment of specific criteria (strong indication for imaging, no abandoned leads, device implant older than 6 weeks), use of a strict protocol with continuous monitoring during imaging, and cooperation between radiologists, radiographers and a supervising appropriately trained (advanced cardiovascular life support [ACLS]) physician or specialist nurse practitioner.17
The effects of device artefact on image interpretability have also been a significant problem with MRI. In a single-centre observational study of a standardised CMR imaging protocol in 111 consecutive cardiac MRI studies in patients with ICDs, Do et al. found that use of a wideband protocol for LGE imaging led to a high proportion of interpretable studies unaffected by artefact (87 %).18 No adverse events (arrhythmias, generator/lead failures) were detected during imaging or up to 6 months’ follow-up.
Nuclear imaging may be of value in selected cases. A study of patients with unexplained non-ischaemic cardiomyopathy and ventricular arrhythmias showed that nearly half had focal abnormal cardiac fluorine-18 fluoro-2-deoxyglucose (FDG) uptake when investigated with fasting PET/CT. Notably, over half of PET-positive patients who underwent CMR imaging had studies negative for LGE; in the rest, LGE and FDG uptake were well correlated. Areas of PET abnormality matched low-voltage scar regions on electroanatomic mapping, which further corresponded with histological analysis.19
Management of ICDs
In patients with ventricular arrhythmias in the context of SHD (i.e. the secondary prevention population), ICD implantation is indicated in almost all cases. While the ICD is effective in preventing sudden cardiac death due to VT or ventricular fibrillation in patients with heart failure, it does not prevent occurrence of VT, and many patients with an ICD will present with one or multiple shocks.20–22 Recurrent ICD shocks have been shown to lead to increased morbidity and mortality, likely a reflection of the progression of underlying cardiac disease.23–25
In patients with recurrent ICD shocks, reprogramming of ICDs by the EP team can help to minimise shocks. The use of overdrive or anti-tachycardia pacing (ATP) to terminate haemodynamically stable VTs before shocks has been shown to be effective, with similar rates of VT acceleration, VT duration, syncope and sudden death when compared to shock only.26 Reprogramming detection zones and detection intervals is a balancing act that involves allowing for self-termination of ventricular arrhythmias against not delaying therapy for symptomatic or haemodynamically unstable arrhythmias.
In patients presenting with ventricular arrhythmias and recurrent device therapies, device interrogation should be performed, with adjustment of rate detection zones and intervals based on the cycle length of clinical VTs; this is in contrast with primary prevention devices, where higher rate cut-offs and longer detection intervals are usually feasible to prevent shocks.27 Finally, the role of inappropriate shocks in recurrent therapies should not be ignored. SVTs represent the most common cause of these, and the use of SVT discriminators can help reduce inappropriate shocks. Onset, stability and morphology criteria help discrimination between SVTs and VTs; this is particularly the case in patients with a history of VT in lower rate zones, where clinical VT characteristics can be accounted for in the discriminator algorithm.28
Medical Therapy
In the structurally normal heart with normal heart function, idiopathic VTs such as outflow tract VT, papillary muscle VT and fascicular VT can be managed with an initial trial of beta-blockers or calcium channel blockers. Although efficacy can be limited, their side-effect profile is relatively favourable. These are used both acutely and for long-term suppression of arrhythmias.
In patients with SHD and VT, antiarrhythmic drugs can be used in conjunction with ICD programming to minimise shocks. Beta-blockers have been shown to decrease mortality in patients with VT, heart failure and reduced EF, and are often used in the absence of contra-indications. However, they are ineffective when used as monotherapy for prevention of VT recurrence.
In a recent meta-analysis, the use of antiarrhythmic drugs led to a 34 % reduction in appropriate ICD therapies;29 the majority of these effects were observed in studies of amiodarone against control medical therapy. Beta-blockers and amiodarone are often used as combination therapy, with improved outcomes and suppression of VT recurrence. This has been shown to be superior to both beta-blockers alone, and sotalol alone.30 Mexiletine, a class IB antiarrhythmic, has been shown in small non-randomised studies to reduce VT recurrence when used as an adjunct to amiodarone in amiodarone-refractory VT,31 and is most commonly used in this setting.
Sotalol has been shown to be safe and effective in reducing mortality and ICD shocks.32 However, given its inferiority in subsequent studies when compared against beta-blockers and amiodarone,30 it is primarily used as a second-line therapy. Finally, ranolazine is an inhibitor of the late inward sodium current initially used in the anti-anginal and anti-ischaemic setting, and subsequently found to be effective in VT suppression in the setting of recurrent ICD shocks refractory to other antiarrhythmic drugs.33 This is currently being investigated in a larger scale population in the Ranolazine Implantable Cardioverter-Defibrillator trial (RAID, NCT01215253).34
However, antiarrhythmic drug use has not been shown to improve survival. In particular, despite well-characterised benefits in VT suppression, amiodarone appears to be associated with increased all-cause mortality.29 Amiodarone use is associated with a high incidence of side effects, primarily affecting the thyroid, lungs, liver and skin;35 patients on amiodarone need to be regularly monitored with blood tests. Significant discontinuation rates (18–38 %) for amiodarone have been noted in multiple trials.30,36–38
Catheter Ablation of Ventricular Tachycardia
Catheter ablation of VT was first described in the 1980s,39 and has since gained an increasingly prominent role in the management of many types of VT.40 With the development of electroanatomic mapping, advances in ablation technology and the development of epicardial approaches, ablation has become an effective intervention in an increasingly broad range of VT aetiologies, and is now deployed increasingly early in the management of recurrent VT.
The impact of catheter ablation has been studied in a variety of causes of VT. In idiopathic VT (outflow tract, fascicular, papillary), ablation can be undertaken in patients intolerant of or refusing medical therapy, in cases where VT has led to reduction in LVEF, or where outflow tract PVCs are found to trigger malignant arrhythmias; procedural success rate is high, with low risk.41
In ischaemic cardiomyopathy, multiple small-scale trials (‘Substrate Mapping and Ablation in Sinus Rhythm to Halt Ventricular Tachycardia’, SMASH-VT; ‘Ventricular Tachycardia Ablation in Coronary Heart Disease’, VTACH) have demonstrated reductions in ICD therapies and greater freedom from VT for patients undergoing ablation and ICD implantation compared to ICD implantation alone.42,43 In the recent ‘Ventricular Tachycardia Ablation Versus Escalated Antiarrhythmic Drug Therapy in Ischemic Heart Disease’ (VANISH) trial, catheter ablation was also found to be superior to escalation of antiarrhythmic therapy in reducing the incidence of a composite primary endpoint of death, VT storm and appropriate ICD shocks.35
In non-ischaemic cardiomyopathies, outcomes are mixed. Studies in idiopathic dilated cardiomyopathy (IDCM) appear to show higher rates of VT recurrence when compared to ablation in ischaemic cardiomyopathy,44 for multiple underlying reasons. Mapping and ablation of IDCM is more challenging: the substrate is often less well defined and patchy, and in locations where ablation is less effective such as intramurally, septally or in the basal anterior LV wall.45 A study of patients with unexplained cardiomyopathy and ventricular arrhythmias who underwent PET/CT showed that a significant proportion had arrhythmogenic inflammatory cardiomyopathy, with diagnoses of cardiac sarcoidosis in 36 %.19 Immunosuppressive therapy was effective in controlling VT and preventing recurrence either as monotherapy or in conjunction with ablation, demonstrating a role for inflammation in the generation and maintenance of ventricular arrhythmias. In these cases, patients may benefit from joint management with other specialties, such as pulmonologists in sarcoidosis, as cardiomyopathy is rarely the sole manifestation of this disease.
The use of combined endo-epicardial mapping and ablation in ARVC has achieved good outcomes,46,47 with significantly less VT recurrence than with endocardial mapping only.48–50 The application of epicardial ablation has recently been extended to Brugada syndrome, where targeting of the right ventricular outflow tract can eliminate the Brugada pattern and prevent recurrence of VT in a subset of cases.51,52
The trend towards earlier ablation appears to be supported by improved outcomes in the literature: in one retrospective study of ischaemic and non-ischaemic cardiomyopathy, ablation within 30 days of first documented VT was associated with significantly higher rates of acute procedural success (defined as noninducibility of VT at end of ablation), and freedom from VT recurrence, although cardiac mortality was not significantly different;53 this is a finding replicated by other studies in the field.42,43,54 Although none of the aforementioned studies have demonstrated a reduction in all-cause mortality with catheter ablation, analysis of patient outcomes from large multicentre registries has demonstrated that freedom from VT recurrence post-procedure is associated with lower all-cause mortality and progression to transplantation.55
Catheter ablation of VT is a complex procedure: management is best undertaken in dedicated units, with integrated multidisciplinary care.3 Some evidence suggests that increased procedural time may result in higher rates of in-hospital mortality.56 As a result, a structured approach to ablation is necessary.
Pre-procedurally, this includes optimisation of patient comorbidities, particularly heart failure with specialist input, withholding anticoagulants and antiarrhythmic medications, and planning for approach of the arrhythmogenic substrate with imaging and preparation of equipment and personnel. Although pre-procedural protocols vary between centres, specific examples of the general approach are available in the literature.57
Intra-procedurally, familiarity with mapping techniques and manoeuvres, ability to appropriately assimilate information, and awareness of limitations and alternatives is essential to prevent the futility of inefficient or ineffectual procedure time.57 The development of modern electroanatomic mapping systems, in combination with pre-procedural imaging, has enabled real-time visualisation of substrates during mapping.58
Post-procedurally, admission to CCU, with multidisciplinary input from CCU staff, EP teams, heart failure specialists, nursing staff and physiotherapists, helps detection of complications and optimisation of post-procedural recovery. Post-procedural testing can help identify patients who may need further interventions.57
Management of VT Storm and Incessant VT
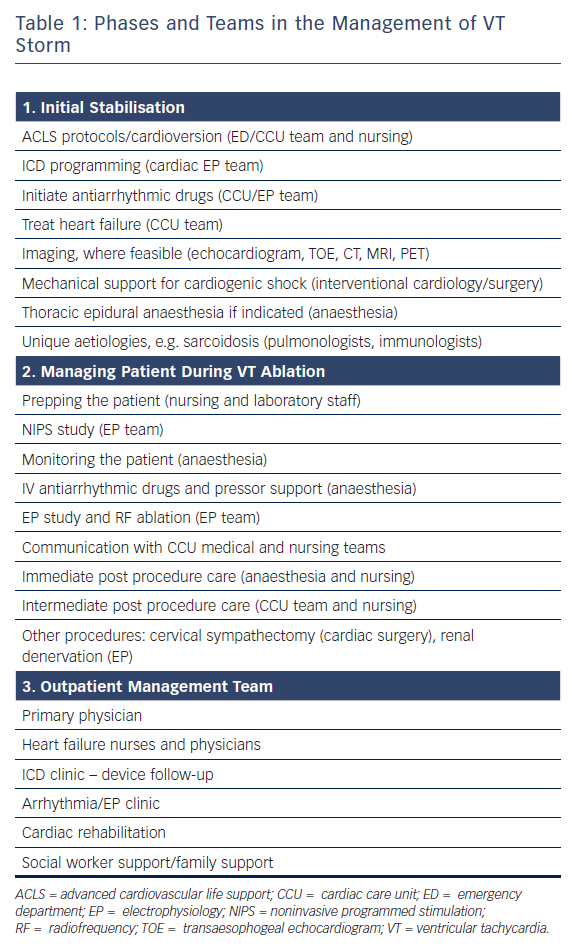
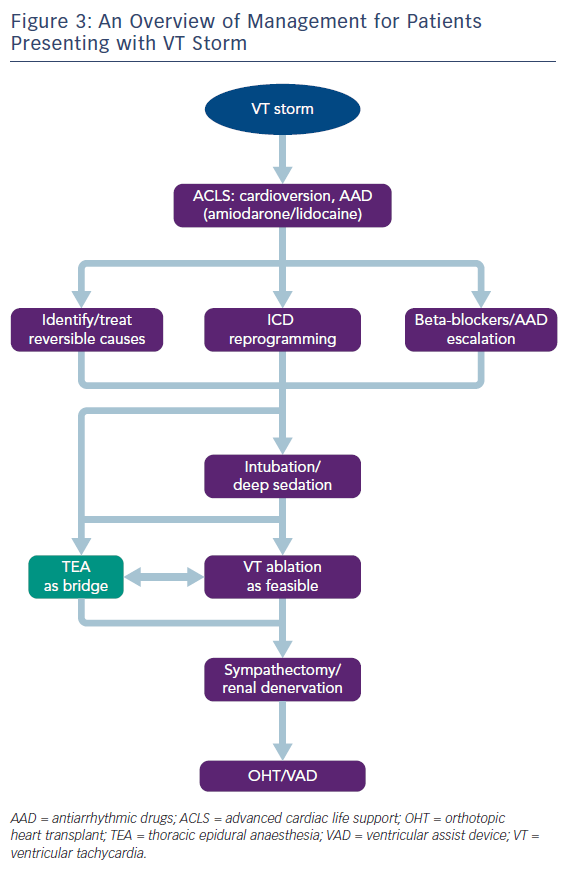
VT storm is defined as three or more separate episodes of sustained VT requiring intervention (such as ICD shock or ATP) within 24 hours. This is a medical emergency requiring prompt intervention with a multidisciplinary team approach to stabilise the patient, initiate therapies and optimise the patient for further interventions (Table 1 and Figure 3). On admission, patients with significant medical comorbidities, or with VT that is not haemodynamically tolerated, should be admitted to a coronary care or intensive care unit.
Initial stabilisation and resuscitation is often carried out by the CCU team following ACLS protocols to stabilise the patient, with cardioversion if necessary. Reversible causes of electrical storm should be sought and corrected where applicable, such as acute ischaemia, electrolyte imbalances, drug-induced proarrhythmia and decompensated heart failure.
ICD Programming and Initiation of Antiarrhythmic Medications
If the patient has an ICD in situ, device interrogation is required to determine the nature of shocks, and to reprogram the device to minimise shocks, through the use of ATP, extending VT detection duration and increasing rate detection thresholds where appropriate. Anti-arrhythmic medications are the first-line therapy in emergency departments and CCUs, as discussed earlier. Amiodarone is most commonly used, along with lidocaine, and in some cases procainamide. Maximising beta-blockade in this situation is important in breaking the cycle of sympathetic stimulation, which initiates VT, and, fuelled by ongoing stress responses from repeated shocks, often provokes further episodes of VT.
Sedation
Sedation in VT storm can be beneficial in reducing sympathetic tone, along with the pain associated with repeated shocks, and is used often. In particular, patients who are haemodynamically unstable with VT may require general anaesthesia with intubation, potentially with mechanical haemodynamic support. However, sedation can lead to further decompensation in the form of severe hypotension in patients who have limited haemodynamic reserve, and must be managed carefully; in addition, prolonged intubation is not ideal.
Thoracic Epidural Anaesthesia and Percutaneous Stellate Ganglion Blockade
As the role of the sympathetic nervous system in the generation and maintenance of ventricular arrhythmias is increasingly recognised, therapies that modulate the sympathetic nervous system, such as cardiac sympathetic denervation and renal denervation have gained prominence in the management of refractory VT.59 However, these procedures cannot be feasibly performed in the acute phase, and are usually reserved for those patients with VT refractory to catheter ablation. Thoracic epidural anaesthesia (TEA), on the other hand, is an intervention that can be more easily instigated acutely: originally used for perioperative pain relief, it also provides sympathetic blockade at the level of T1–T4, and has been shown to be effective in controlling VT storm.60,61 The largest study to date of patients presenting with VT storm or incessant VT found that TEA could be safely performed, with a response seen in over half of patients.62 In some patients where response was observed, it was possible to wean off antiarrhythmic medications, or extubate patients. Thus, TEA can be employed as a substitute for deep sedation, and can be an effective bridging therapy allowing for stabilisation before further definitive therapy, such as catheter ablation (Figure 3). The authors suggest that TEA should be considered in patients without other reversible factors for VT storm, no contraindications to thoracic epidural catheter placement (e.g. infection or continuous therapeutic anticoagulation required), and where VT is not controlled despite the use of two or more antiarrhythmic medications.
Percutaneous stellate ganglion blockade is another autonomic modulatory intervention that can be performed at the bedside. Although descriptions of its efficacy have primarily taken the form of case reports, a recent meta-analysis showed beneficial effects in reduction of ventricular arrhythmia episodes and defibrillation, allowing patients in some cases to proceed to further definitive treatment such as ablation or transplantation.63
Mechanical Haemodynamic Support Devices
The use of mechanical haemodynamic support may be necessary to maintain end-organ perfusion in presentations with VT storm. It is essential to seek input from heart failure specialists and cardiac surgeons in assessing and planning to use haemodynamic support options. Available devices include intra-aortic balloon pumps (IABP), percutaneous left ventricular assist devices (pLVAD) such as the Impella and TandemHeart systems, and extracorporeal membrane oxygenation (ECMO). The IABP has historically been the most commonly used of these, although pLVAD and ECMO, which give additional support, are being increasingly employed in specialised centres in recent years.
Haemodynamically unstable VT storm is not the only setting in which mechanical haemodynamic support is used in VT. It is also used in preparation for ablation in patients with poor LVEF at baseline, when VT induction will likely result in acute haemodynamic decompensation (AHD).64 The need for a large arterial cannula for maximal output in the TandemHeart system necessitates arterial cutdown and insertion by an interventionalist with experience in use of the device. ECMO presents an even greater challenge, requiring coordination from intensivists, perfusionists and cardiac/vascular surgeons to initiate and monitor its use.
Risk Stratification and Patient Management for Catheter Ablation of VT
Catheter ablation in the setting of VT storm has been shown to suppress acute recurrence and stabilise the patient in the short term, even if the procedure is not completely successful;65 in longer term follow-up, procedural success is associated with reduced VT storm and improved survival, although risk of recurrence remains high.65,66 In the VANISH trial, catheter ablation led to reduced occurrence of VT storm compared with escalation in antiarrhythmic therapy.35 Combined endocardial and epicardial mapping and ablation can be used in this setting, in patients who satisfy criteria suggestive of epicardial substrate or circuit.67
As VT ablation carries high risk of post-procedural morbidity and mortality, pre-procedural risk assessment is important. This should aim to guide selection of patients not appropriate for ablation, and to identify high-risk cases that may require more aggressive optimisation with involvement of intensivists and perfusionists for prophylactic haemodynamic support.
The use of existing models can be useful to predict medium- to long-term survival in patients being considered for catheter ablation, which can then be weighed against the risk/benefit of proceeding. The Seattle Heart Failure Model, a commonly used and widely available tool for estimating mortality in patients with heart failure, has been shown to accurately identify those at high risk of mortality within 6 months of VT ablation when modified with the inclusion of risk modifiers for VT storm and ICD shocks. This not only acts as a useful tool to justify not undertaking ablation in some patients, but also aids discussions with patients and families regarding expectations for prognosis.68
A major risk of performing VT ablation is periprocedural acute haemodynamic decompensation (AHD), defined as persistent hypotension that requires mechanical support or procedure discontinuation. This is often secondary to the severity of the underlying cardiac disease, and aspects of ablation, such as induction of anaesthesia, repeated induction and termination of VT, and fluid overload from catheter irrigation. AHD is a particular concern for patients with heart failure and multiple comorbidities – common findings in patients undergoing ablation – although identification of those most at risk has not always been simple.
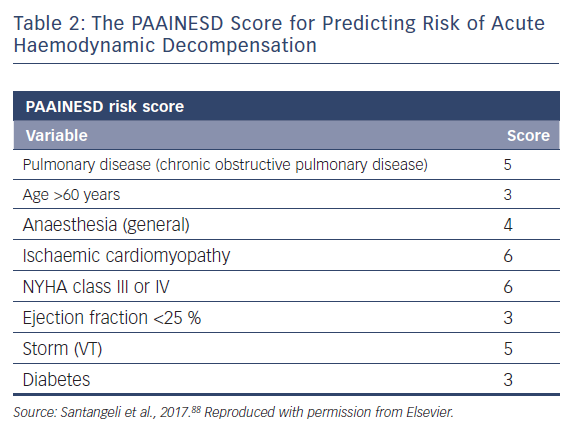
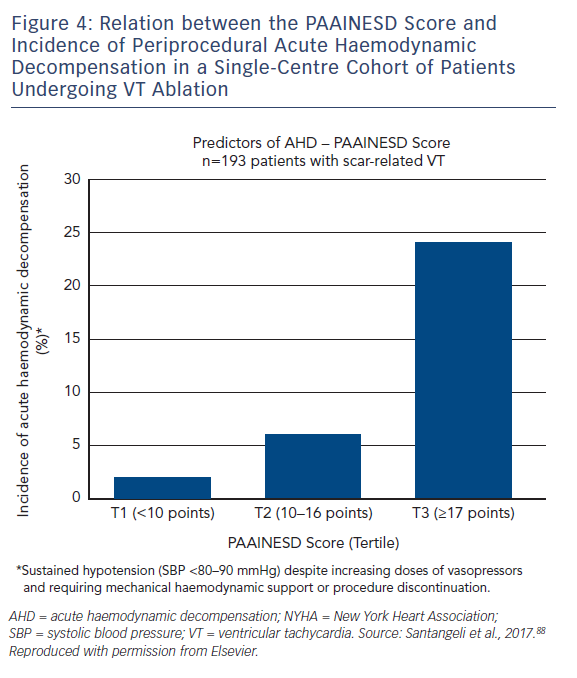
A single-centre study characterised the incidence of and risk factors for AHD in 193 patients undergoing catheter ablation.69 This occurred in 11 % of cases, and eight clinical variables were significantly associated with increased risk: age over 60 years; use of general anaesthesia; ischaemic cardiomyopathy; more severe heart failure (New York Heart Association [NYHA] class III/IV); reduced LVEF; presentation with VT storm; diabetes; and chronic obstructive pulmonary disease. Based on the associated odds ratios, the authors developed the PAAINESD score, a 35-point scale divided into three risk categories (Figure 4 and Table 1). In the study, AHD occurred in 2 %, 6 % and 24 % of patients in each category, respectively. Additionally, patients with AHD in the study had significantly higher mortality at 6 months and 1 year, with a tendency towards higher 30-day mortality. This was supported by a subsequent large-scale multicentre retrospective application of the PAAINESD score to 2,061 patients undergoing VT ablation, using data from the International VT Ablation Center Collaboration Group (IVTCC). PAAINESD scores were significantly higher in cases of early mortality (within 31 days post-ablation), compared with survivors and deaths beyond 31 days.70 The developers of the PAAINESD score thus suggest that it could be used to predict AHD and early mortality in patients undergoing catheter ablation, which could be minimised not only with appropriate patient selection, but also through more aggressive pre-procedural optimisation and use of prophylactic haemodynamic support.
In support of this, one nonrandomised study investigating the role of pre-emptive implantation of pLVAD (Impella, TandemHeart) before ablation compared with rescue pLVAD reported similar PAAINESD scores in the pre-emptive and rescue pLVAD groups, but a significantly higher 30-day mortality rate in the rescue pLVAD group (58 %), compared against both the pre-emptive pLVAD (4 %) and no-pLVAD (2 %) groups.71 Similarly, in a separate retrospective uncontrolled observational study, the use of ECMO as rescue haemodynamic support was associated with a high mortality rate (overall 76 %).72
The PAAINESD score may also help to improve quantification of risk in patients who have risk factors for early mortality, but may actually represent lower risk candidates for ablation. Surveys of centres offering VT ablation and analyses of registries indicate that the rate of ablation is lower in groups such as the elderly and those with severe heart failure.73 Data suggest that ablation often occurs later in the management pathway, and thus likely later in the disease course for elderly patients, with a preference for escalation of antiarrhythmic medications.74 Rates of elective VT ablation in the elderly tend to be lower than in younger populations.75 Following on from this, ablation later in disease, in the emergency setting of VT storm or delaying ablation in presentations with VT storm may increase procedural risk and adversely affect outcomes.54
Such issues are highlighted by IVTCC group registry analyses that have investigated safety and efficacy outcomes, firstly in elderly patients (over 70 years) compared with younger patients,76 and secondly in severe heart failure patients (NYHA class IV compared with NYHA class II/III).77 In general, in-hospital and 1-year mortality were higher in elderly and NYHA IV patients, who overall represented a higher risk group, although acute procedural success and complication rates were not significantly different. Notably, in both studies, rates of successful ablation with long-term VT-free survival were not significantly different between groups. In addition, long-term VT-free survival translated to improved overall survival in elderly/severe heart failure patients compared with patients in the same group with VT recurrence. These results are consistent with smaller scale studies of elderly patients undergoing VT ablation, and subgroup analyses of SMASH-VT and VANISH.35,42,74
These studies demonstrate that VT ablation can be performed safely in appropriately selected patients with high-risk features. It is important to note that freedom from VT recurrence resulted in significantly better overall survival than in patients where VT recurred. This highlights an area of risk stratification that might benefit from further study: the identification of patients who are most likely to respond to VT ablation, with low rates of VT recurrence, and who therefore have the most potential for survival benefit.
Further Procedures
Although ablation procedures have a relatively high success rate, a significant number of VTs remain refractory to ablation. In such cases, further interventions may be considered. First of all, repeat ablation may be indicated. If the original ablation procedure involved only endocardial mapping and ablation, a repeat procedure with epicardial mapping and ablation may be able to prevent further VT.
In some patients, efforts at ablation may have been hindered by difficulties in accessing the substrate, such as for VT originating from the interventricular septum. In such instances, interventions such as transcoronary ethanol ablation may be effective.78
Traditional epicardial access may be hindered by the presence of pericardial adhesions. Here, surgical epicardial access gained with the assistance of cardiothoracic surgeons may be a safe alternative.79
Autonomic modulation procedures may be indicated in the setting of ongoing refractory VT. Surgical cardiac sympathetic denervation can significantly reduce the incidence of ICD shocks in refractory VT.80 Renal denervation has also been shown to prevent VT recurrence in small case series.59 VT ablation may be used as a bridging procedure to insertion of left ventricular assist devices or cardiac transplant, in which case the procedure and post-operative care should be undertaken with involvement from transplant teams and cardiac surgeons.
In some cases, the role of further interventions may be limited or ablation may have been undertaken as a palliative procedure, to minimise the burden of distressing ventricular arrhythmias. It is important that in cases where these situations are anticipated, that clear discussions with patients and relatives are held in advance to explain the rationale behind clinical decision-making and to determine the wishes of the patient while they are able to communicate them. Discussion regarding measures such as device deactivation should be approached in advance.81 It may be useful to discuss with palliative care teams before procedures, and for them to review and meet the patient and family, to smooth the process of transition to supportive care when this is appropriate.
Outpatient and Home Care
It is difficult to predict the long-term outcomes of VT ablation in individual patients. Estimates of VT recurrence and mortality presented in the literature are based on a wide range of studies that have investigated outcomes of ablation in a variety of VT aetiologies with differences in prior therapy, number of procedures and ablation techniques used.82 In general, 1-year recurrence rates are approximately 30 % to 43 %; 2-year recurrence rates are around 50 %.35,43,44,55,83 Future studies may give more accurate estimates of outcomes in specific populations of patients with VT.82
Recurrent admissions with heart failure are common in patients with SHD, although possibly reduced after ablation, and likely represent progression of disease status.83 The 1-year mortality rate post-ablation is reported at about 15–20 %; again, this varies depending on aetiology and VT-free survival status, and is often reported as a composite with transplant-free survival.55,84 Much of this is driven by progressive heart failure and is not necessarily surprising given the knowledge that ICD shocks are associated with increased risk of mortality from heart failure.25
Patients require ongoing care and regular review after discharge. Care in the community is primarily conducted by the patient’s primary care physician and general cardiologist, heart failure specialist nurses and cardiac device technicians, but further specialist input from heart failure and electrophysiology teams may be required. The use of home monitoring systems in patients with implanted devices may be useful to monitor for VT recurrence. Patients may require re-do ablations, further interventions, or cardiac transplantation, as discussed above.
Finally, psychological support for the patient is important: although life-saving, repetitive shocks can exacerbate anxiety and reduce quality of life, at least in the short term, as well as increasing subsequent risk of death.85,86 It is important to support the patient to build confidence, and to allow patients to maintain a good quality of life despite this.
Conclusion
Management of patients with ventricular arrhythmias is complex. In outpatient or non-specialist settings prompt referral is important, and this is achieved by ensuring good links between community care givers and hospital teams. In-hospital management requires multispecialty input in a dedicated specialist setting. Co-ordination of care, in our hospital by the coronary care unit attending cardiologist and team, ensures that appropriate additional specialist input is sought, and investigations and interventions can be coordinated.
Catheter ablation has assumed an increasingly prominent role in the treatment of VT. Although early referral of patients for catheter ablation is desirable, appropriate patient selection is required using existing risk-stratification schemes. Further studies will better quantify the timing and benefits of catheter ablation in specific subpopulations of patients with VT.
Finally, at the centre of care remains the patient, for whom recurrent arrhythmias and worsening heart failure often bring significant morbidity and mortality. It is important to discuss options, risks and prognosis to allow patients to make informed choices about their care. The combination of a patient-centred approach with modern treatment modalities and appropriate specialist care is vital to ensuring optimal outcomes in patients presenting with VT.
Finally, at the centre of care remains the patient, for whom recurrent arrhythmias and worsening heart failure often bring significant morbidity and mortality. It is important to discuss options, risks and prognosis to allow patients to make informed choices about their care. The combination of a patient-centred approach with modern treatment modalities and appropriate specialist care is vital to ensuring optimal outcomes in patients presenting with VT.
Clinical Perspective
- Modern management of patients with ventricular arrhythmias is complex, and requires a multidisciplinary team approach in experienced units to ensure optimal outcomes.
- Appropriate initial investigations and prompt referral to specialist care are key to optimal management of ventricular tachycardia, as well as establishing underlying aetiology, which has significant implications for management.
- Medical therapy may be effective in some cases, but may have significant side effects. ICD therapy prevents sudden cardiac death, but can lead to recurrent shocks, which results in increased morbidity and mortality, likely an indication progression of underlying disease.
- Radiofrequency catheter ablation has gained an increasingly prominent role in the management of ventricular arrhythmias, although further studies are required to define the risk and outcomes of procedures in individual patients.
- We discuss and give an overview of the management of patients presenting with ventricular tachycardia storm, including the evolving role of neuromodulation.