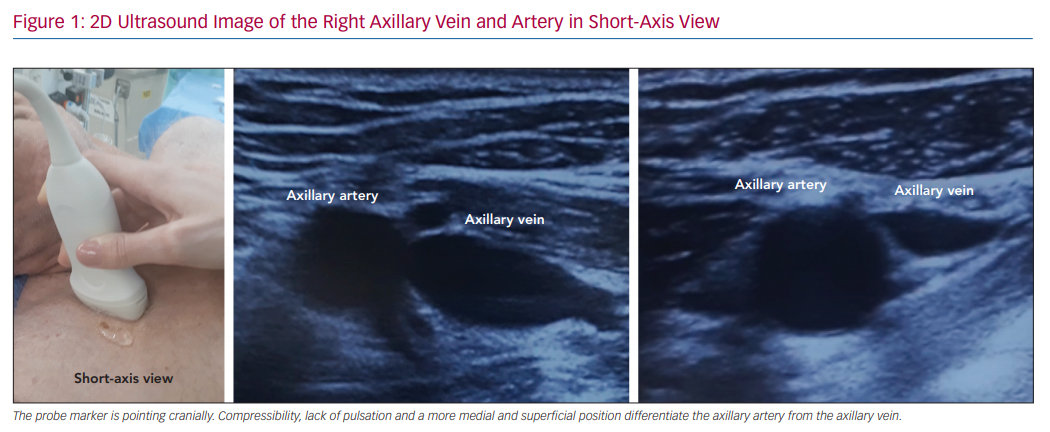
Cardiac implantable electronic devices (CIEDs), including permanent pacemakers (PPMs), ICD and CRT devices, are the mainstream therapy for many potentially lethal heart conditions, such as advanced atrioventricular block or sustained ventricular tachycardia or fibrillation. CIEDs can be implanted through endovascular or epicardial routes, with the former used the most because it is less invasive and provides better pacing thresholds.1–3
Since the first successful insertion of a temporary transvenous endocardial lead through the brachial vein by Furman and Schwedel in 1959,4 many technical advancements have been described, culminating in the widespread use of the procedure.5 It is projected that the global number of PPMs implanted annually will be 1.43 million by 2023.6
A European Heart Rhythm Association survey showed that cephalic vein dissection and blind subclavian vein puncture are the preferred techniques for the implantation of CIED leads in European centres.5 However, these two techniques are associated with variable success and complications rates that can be reduced by using imaging guidance.
Cephalic access has been used as a route for endocardial lead implantation since 1960.7,8 Despite its relative safety, by avoiding central venous puncture, the method is associated with high failure rates and longer procedure times.9 Cardiac lead insertion is also highly dependent on venous anatomy, trajectory, calibre and operator skills,10 which culminates in failure rates ranging from 10% to 70%.11–15
Conversely, subclavian vein puncture is a highly successful approach, but requires central venous puncture, the complications of which, although uncommon, can be potentially fatal.16,17 In addition, leads implanted through subclavian puncture are more susceptible to long-term dysfunction secondary to subclavian crush syndrome.18–22
To address these problems, punctures guide d by fluoroscopy, venography and ultrasound (US) have emerged as feasible
and reproducible alternatives to increase procedure success and safety.7,23–28 Another relatively new method that has received increasing attention is axillary vein puncture, the first application of which in CIED implantation was described by Byrd in 1993.29
By anatomical definition, the axillary vein is a continuation of the brachial vein, originating at the lower margin of the teres major muscle and terminating at the lateral margin of the first rib. The extrathoracic location of the axillary vein and its distance from the first rib explain the lower rates of pneumothorax, haemothorax, inadvertent arterial puncture and subclavian crush syndrome following axillary vein puncture. In addition, the axillary vein has a large calibre, allowing multiple punctures or multiple lead insertions through the same puncture.18
Despite these benefits, lead insertion using the axillary vein remains uncommon in many centres, primarily due to the lack of proper training and concerns with a supposed long learning curve.
Ultrasound-guided Venous Puncture
US-guided puncture allows direct visualisation of the vessels and surrounding structures. Therefore, the puncture is safer and less time consuming.30,31 Since 2001, US-guided central catheter placement has been recommended by the Agency for Healthcare Research and Quality as one of the 11 fundamental practices to improve procedural safety.32 However, this recommendation applies mostly to the jugular vein, because there was not enough evidence supporting US-guided axillary vein puncture, especially during CIED implantation.33–35
The first description of US-guided axillary vein location was reported by Shregel et al. in 1994.36 This was followed by the first real-time US-guided axillary vein puncture, initially using the short axis, by Nash et al. in 1998 and, posteriorly, using the long axis by Sandhu in 2004.25,37 Subsequently, many reports have suggested that this method is associated with a short time to obtain central venous access, a reduced number of puncture attempts and low complication rates.25,31,38–40
Some ultrasound characteristics make the axillary vein easily distinguishable from the axillary artery and feasible for clinical use, such as the lack of pulsation, a more medial and superficial position and external pressure compressibility (Figure 1). Ultrasound scanning also allows evaluation of vein patency prior to pocket creation, which may be useful in patients with prior thoracic surgery, radiotherapy exposure or dialysis catheters.39
Another particularly relevant advantage of US is the detection of complications, such as pneumothorax, earlier than with radiological control. Whereas the presence of pleural sliding is the most important finding in a normal aerated lung (‘seashore sign’ using the M-mode), the lack of pleural sliding and the presence of parallel horizontal lines above and below the pleural line (‘barcode’ or ‘stratosphere sign’ using the M-mode) are indicative of pneumothorax.41
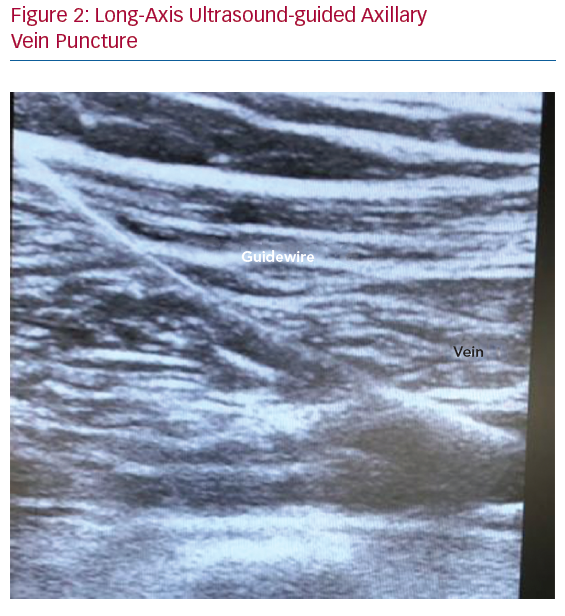
In terms of the US-guided axillary puncture, clear needle visualisation is a crucial step to enable proper puncture, avoiding damage to adjacent structures such as vessels, nerves and pleura. In cross-sectional images, the needle tip can be seen as a highly echogenic spot with surrounding artefacts caused by the scattering of the ultrasound beam, which is less easily visible within the heterogeneous appearance of body tissue. In the out-of-plane needle approach, the needle shaft is not visualised, and indirect evidence of vein compression may be seen (Figure 2).42
The probe most used to guide axillary vein puncture is the vascular high-frequency linear array probe (5–10 MHz), which provides a high-resolution image. If a more medial puncture is desired, a microconvex linear array probe with a smaller footprint may be an alternative to deal with the acoustic shadowing from the overlying clavicle.43 A more medial puncture offers a less deep and steep puncture angle and reduces the risk of brachial plexus injury, the incidence of which in axillary puncture varies from 0% to 1.3%.44 Symptoms related to brachial plexus injury can be attributed to direct trauma of the nerve by the needle due to repeated puncture attempts in a too lateral
position or to brachial plexus block induced by lignocaine.44
Compared with fluoroscopy and venography, US-guided puncture has some advantages, such as a faster effective learning process and no requirement for extra X-ray exposure, additional peripheral access or contrast injection. These features may potentially avoid renal function impairment, allergic reactions and venous spasm related to the use of contrast.45 Furthermore, the use of venography for axillary vein puncture is limited by the inability to estimate the depth of the puncture with this method.
Scientific Evidence Comparing CIED Lead Insertion Through Axillary Vein Puncture With Other Techniques
Calkins et al. were one the first groups, in 2001, to undertake a randomised clinical trial evaluating venography-guided extrathoracic subclavian puncture versus cephalic vein dissection in 200 participants who had undergone PPM or ICD lead implantation.46 The extrathoracic subclavian group had higher success (99% versus 64%; p<0.001), shorter time to obtain central venous access (mean ± SD: 10 ± 8 minutes versus 25 ± 17 minutes; p<0.01), shorter total procedural time (86 ± 22 minutes versus 98 ± 35 minutes; p<0.01) and lower blood loss (55 ± 13 ml versus 115 ± 107 ml; p<0.01), but there was no significant difference in early complication rates between the two groups (6% versus 11%; p=0.2).46
In 2016, Lui et al. published the results of another randomised control trial comparing fluoroscopy-guided axillary vein puncture to standard blind subclavian vein puncture in 247 CIEDs.47 Comparisons of axillary and subclavian punctures revealed similar first-puncture attempt success (68.4% versus 66.1%, respectively; p=0.597) and overall success rate (95.7% versus 96%, respectively; p=0.845). Despite these similarities, the time to perform the puncture was shorter in the subclavian puncture group (46 ± 14 versus 28.7 ± 14 s; p<0.001), but, over a mean follow-up period of 24.1 ± 7.4 months, the complication rate was lower in the axillary puncture group (1.6% versus 8.2%; p=0.016). In terms of severe complications, three cases of pneumothorax and two of subclavian crush syndrome were reported with subclavian access.47
In 2018, Liccardo et al. compared PPM and ICD lead insertion through US-guided axillary vein puncture to anatomical landmark-guided subclavian puncture in 174 participants.38 Before starting the comparative study, a training phase (60 axillary cases) was performed, during which an axillary success rate of 69% was achieved. During the randomised phase, no difference in success rate was reported (91.4% versus 94.8% in the axillary and subclavian groups, respectively). Over a mean follow-up period of 18 ± 6 months, lead complications were similar in both groups (2.6% versus 5.2%; p=0.664), with two cases of pneumothorax (3.4%) requiring thorax drainage and longer hospitalisation length of stay in the subclavian group. Axillary puncture was considered a safe and efficient alternative to the standard subclavian access for CIED implantation.38
In contrast to the finding of lower success in the initial training phase noted above, Squara et al. demonstrated excellent outcomes for a self-taught axillary technique from the first case.44 That study was a prospective randomised trial comparing self-learned fluoroscopy-guided axillary vein puncture with cephalic vein dissection in 74 participants undergoing PPM implantation. Similar venous access success (81.1% versus 75.7% for axillary vein puncture and cephalic vein dissection, respectively; p=0.57) and 30-day complication rates (13.5% versus 10.8%; p=0.71) were obtained, with shorter venous access time (5.7 minutes versus 12.2 minutes; p<0.001), total procedural time (34.8 minutes versus 42 minutes; p=0.043) and X-ray exposure (1,463 versus 1,013 mGy·cm2; p=0.12) in the axillary group.44 These results were quite consistent throughout the study, independent of the number of cases performed by each operator. Based on these findings, the authors highlighted that one of the particular advantages of the axillary vein is its possible use as a bail-out alternative when the cephalic vein is absent or has an unsuitable calibre, avoiding intrathoracic puncture.44
Esmaiel et al. also reported their experience with US-guided axillary vein puncture in 403 consecutive patients who underwent a PPM implantation between 2012 and 2015.48 In that study, a success rate of 99.2% was obtained, with a mean number of 1.18 venepuncture attempts per patient and a mean time of 2.24 minutes to obtain central venous access. No access-related complication was reported.48 However, because that study was a retrospective, observational, single-centre and single-operator study, its external validity could be questionable.
An interesting point from the study by Esmaiel et al. is that the authors had described puncturing the vein from inside the pocket incision using a sterile covered probe.48 According to Esmaiel et al., this puncture is performed 1–2 cm medial to the deltopectoral groove, therefore more medial and slightly cranial than the standard incision for a cephalic cutdown.48 We prefer to puncture first and to create the pocket after because, with this technique:
- we can incise the skin in a medial position, far from the axillary region, in a location that we judge more comfortable to the patient;
- the puncture site does not get restricted to the incision area, which enables us to more easily change the position of vascular linear probe (e.g. from the short to long-axis or from lateral to medial), scanning the entire vein; and
- we avoid applying gel inside the pocket, even sterile gel, due to concern of infection.
Despite these arguments, in obese patients puncturing from inside the pocket could be preferable because this technique facilitates vein visualisation by obviating imaging impairment due to the deep layer of subcutaneous fat.
In our practice, in chronological order, we first puncture the vein and insert the 0.035" guidewire. Second, we make the skin incision and build the pocket in a location that we judge more comfortable to the patient. Third, we dissect the tissues until we identify the 0.035" J-wire, which we then pull to the subcutaneous or submuscular space (i.e. subcutaneously in case of subcutaneous pocket and submuscularly in case of a submuscular pocket).
A 2006 prospective non-randomised study comparing PPM lead implantation by US-guided axillary puncture versus cephalic
vein dissection evidenced similar success rates for the two approaches (88% versus 87%, respectively), with shorter lead placement time in the axillary group (8 minutes versus 12 minutes; p<0.05).31 It was also reported that the operators achieved lead placement times with US-guided axillary puncture that were equivalent to those for cephalic dissection after 25 cases; however, once the US-guided technique was mastered, the operators had faster lead placement times with this method than with cephalic dissection. In this analysis, independent predictors of lead placement time were BMI, operator experience, initial strategy (ultrasound versus cephalic) and number of procedures.31
Regarding predictors of late lead complication, Chan et al. reported that, over a mean follow-up period of 73.6 ± 33.1 months, subclavian vein puncture instead of axillary vein puncture was the only independent predictor of pacemaker lead failure (HR 0.26; 95% CI [0.07–0.95]; p=0.042).49 In this analysis, the success rate was significantly lower in the cephalic group (78.2%) than in the venography-guided axillary puncture or blind subclavian puncture groups (97.6% and 96.8%, respectively; p<0.001).49 In addition, over a medium-term follow-up period (mean 45 ± 10 months), ElJamili et al. showed that, even in patients under oral anticoagulation or antithrombotic therapy, US-guided axillary puncture presented no postoperative complications and achieved a success rate of 95.78%, with the guidewire insertion time reaching a plateau after 15 patients.50
Considering all these studies, US-guided axillary vein puncture has a success rate ranging from 80% to 99%.25,38–40 Compared with other available access routes, this rate appears better than that reported for cephalic vein dissection (64–87%)31,44,46,49 and similar to that reported for venography-guided axillary puncture (90–98%),10,28,49,51 fluoroscopy-guided axillary puncture (61–98%)10,18,22,23,44,47 and even blind subclavian puncture (94–96%).23,38,47,49
Aiming to fill the evidence gap in the comparison between US-guided axillary puncture and cephalic vein dissection, as well as to provide a strong scientific basis for the use of US-guided axillary vein puncture as the standard technique for CIED implantation, Tagliari et al. recently published the results of the first randomised clinical trial comparing these two approaches during PPM and ICD lead implantation.52 In that trial, the superiority of the US-guided axillary approach was demonstrated in terms of success rate (97.7% versus 54.5%; p<0.001), time to obtain central venous access (5 minutes versus 15 minutes; p<0.001) and total procedural time (40 minutes versus 51 minutes; p=0.010), with no increase in complication rate.52
Conclusion
CIEDs are a widely used, life-saving therapy for different heart rhythm conditions. Because of potential failures or complications of standard implant practices (i.e. cephalic dissection and subclavian vein puncture), alternative techniques have emerged. Among these, US-guided axillary puncture stands out because of its high success rate, associated with a low incidence of complications and short learning curve. In addition, this techniques aligns with the new trend to use US for safer vascular access in different contexts. The article by Tagliari et al. will hopefully contribute to shedding some light on this issue, and possibly to changing standard approaches.52
Clinical Perspective
- Despite not being the standard approach in many centres, axillary vein punctures guided by fluoroscopy, venography and ultrasound have emerged as feasible alternatives for the implantation of cardiac implantable electronic devices (CIEDs).
- Lead insertion through axillary vein puncture is associated with a short learning curve and procedural time.
- Ultrasound-guided axillary vein puncture has a high success rate with a low complication rate, which could make it the preferred approach for the implantation of CIEDs.